Synthesis method of hydroxyl-terminated liquid fluororubber modified polyurethane modulus gradient material
A technology of liquid fluororubber and synthesis method, which is applied in the field of synthesis of polyurethane modulus gradient materials, can solve the problems of non-overlapping functional areas, and achieve the effects of good product quality, easy reaction control, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
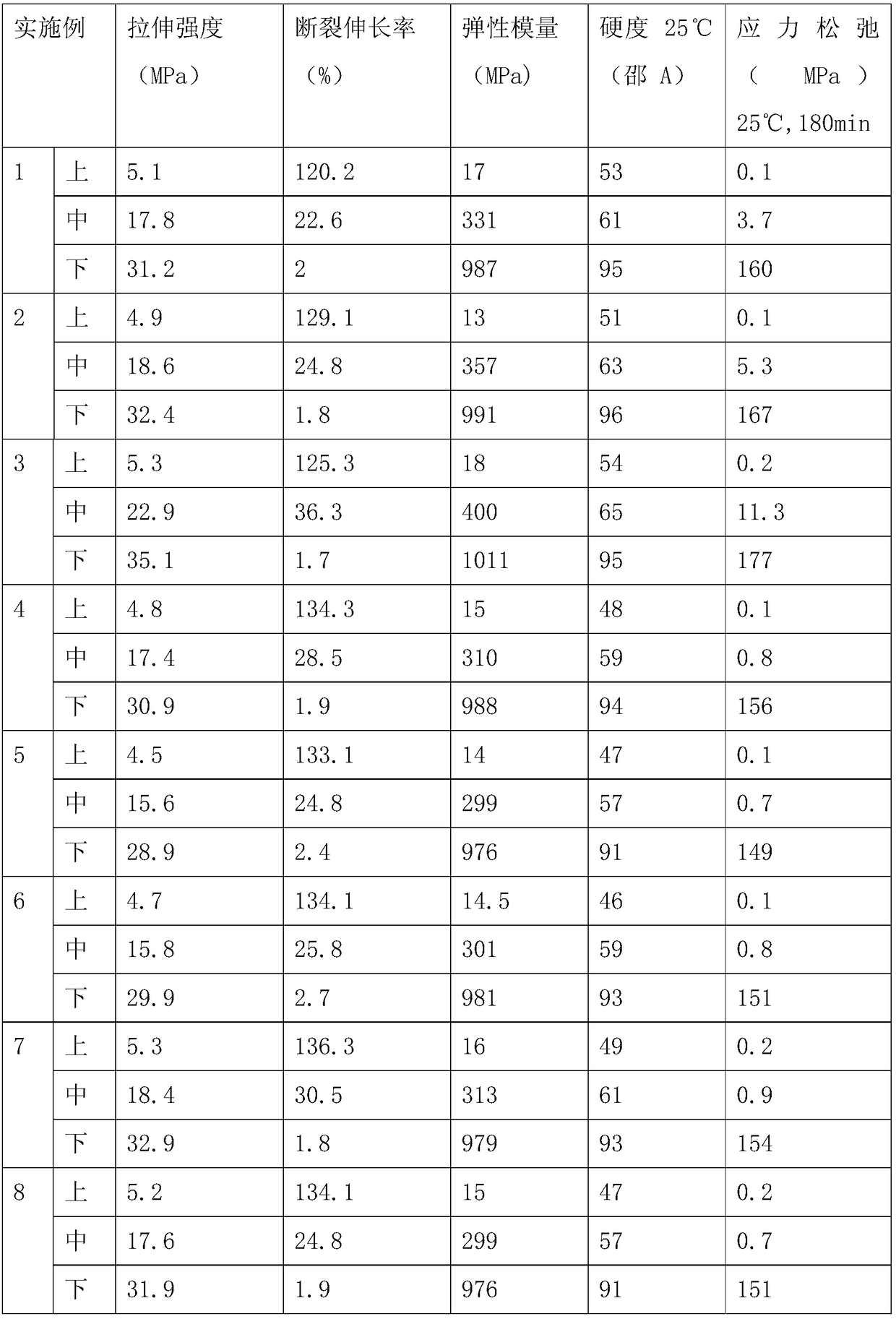
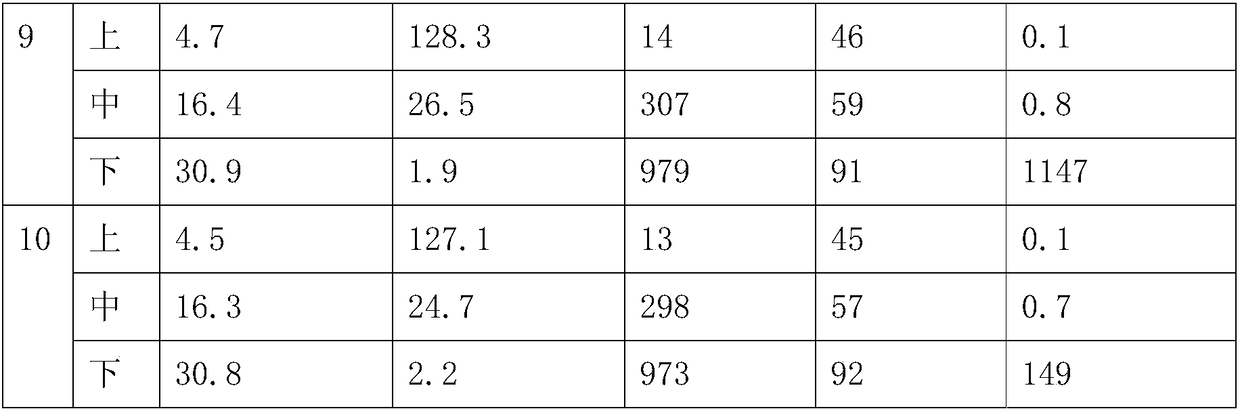
Embodiment 1
[0023] Add 100g of polyoxypropylene glycol (PPG 1000) and 5g of hydroxyl-terminated liquid fluororubber (vinylidene fluoride-hexafluoropropylene copolymer) into a 500mL three-necked flask, put on a thermometer, stirrer, 105 ° C, vacuum -0.1 Dehydration under reduced pressure at MPa. After 3 hours of dehydration, the temperature began to drop. After the temperature dropped to room temperature, 27g of toluene diisocyanate (2,4-TDI) was added, and 0.7g of dibutyltin dilaurate was added. The reaction was carried out at room temperature for 0.5h and at 85°C for 2.5h to obtain polyurethane prepolymer. Continue to add 0.66g of N,N-dimethylbenzylamine, 13.2g of anhydrous acetone and 13.2g of bisphenol A diglycidyl ether to the reaction system, vacuum defoaming at 60°C and stir evenly before use. Turn on the computer-controlled syringe pumps (A, B) with two dosing. Add 60ml of polyurethane prepolymer mixed with catalyst to the A syringe pump, and fill the B syringe pump with 60ml of a...
Embodiment 2
[0026]Add 100g of polyoxypropylene glycol (PPG 1000) and 15g of hydroxyl-terminated liquid fluororubber (vinylidene fluoride-hexafluoropropylene copolymer) into a 500mL three-necked flask, put on a thermometer, stirrer, 105 ° C, vacuum -0.1 Dehydration under reduced pressure at MPa. After 3 hours of dehydration, the temperature began to drop. After the temperature dropped to room temperature, 29g of toluene diisocyanate (2,4-TDI) was added, and 0.77g of dibutyltin dilaurate was added. The reaction was carried out at room temperature for 0.5h and at 85°C for 2.5h to obtain polyurethane prepolymer. Continue to add 0.72g of N,N-dimethylbenzylamine, 14.4g of anhydrous acetone and 14.4g of bisphenol A diglycidyl ether into the reaction system, vacuum defoaming at 60°C and stir evenly before use. Turn on the computer-controlled syringe pumps (A, B) with two dosing. Add 60ml of polyurethane prepolymer mixed with catalyst to the A syringe pump, and fill the B syringe pump with 60ml o...
Embodiment 3
[0029] Add 100g of polyoxypropylene glycol (PPG 1000) and 20g of hydroxyl-terminated liquid fluororubber (vinylidene fluoride-hexafluoropropylene copolymer) into a 500mL three-necked flask, put on a thermometer, stirrer, 105 ° C, vacuum -0.1 Dehydration under reduced pressure at MPa. After 3 hours of dehydration, the temperature began to drop. After the temperature dropped to room temperature, 30g of toluene diisocyanate (2,4-TDI) was added, and 0.8g of dibutyltin dilaurate was added. The reaction was carried out at room temperature for 0.5h and at 85°C for 2.5h to obtain polyurethane prepolymer. Continue to add 0.75g of N,N-dimethylbenzylamine, 15g of anhydrous acetone and 15g of bisphenol A diglycidyl ether into the reaction system, vacuum defoam at 60°C and stir evenly before use. Turn on the computer-controlled syringe pumps (A, B) with two dosing. Add 60ml of polyurethane prepolymer mixed with catalyst to the A syringe pump, and fill the B syringe pump with 60ml of aroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com