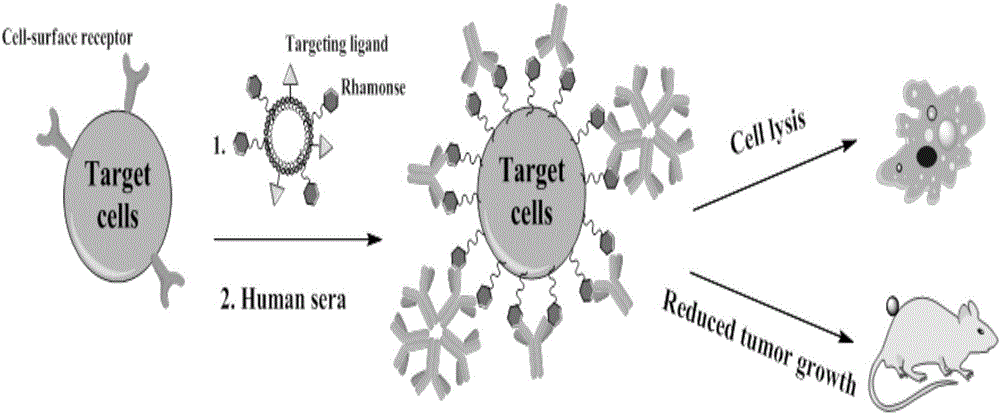
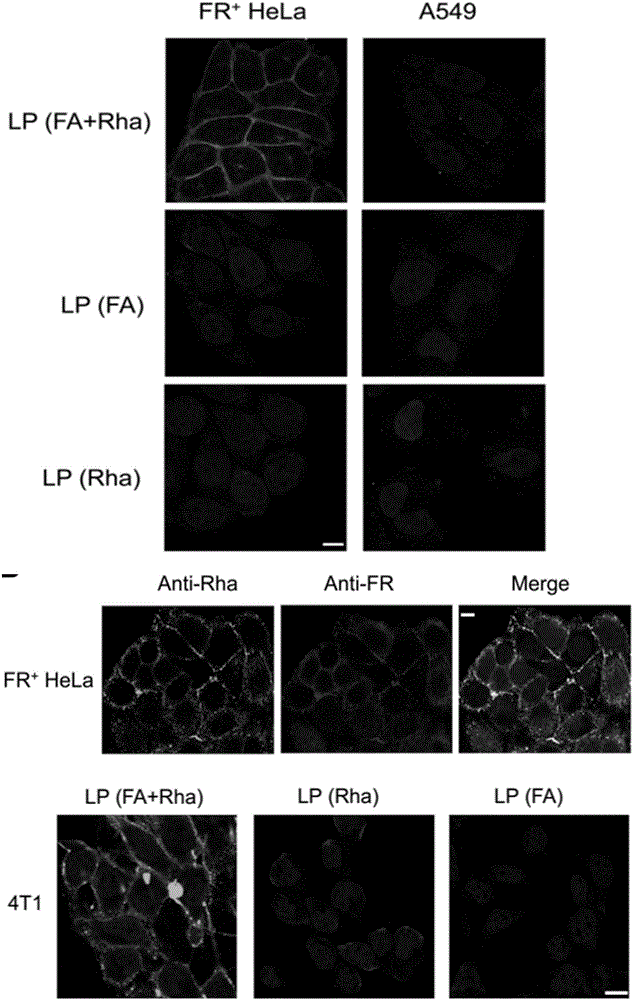
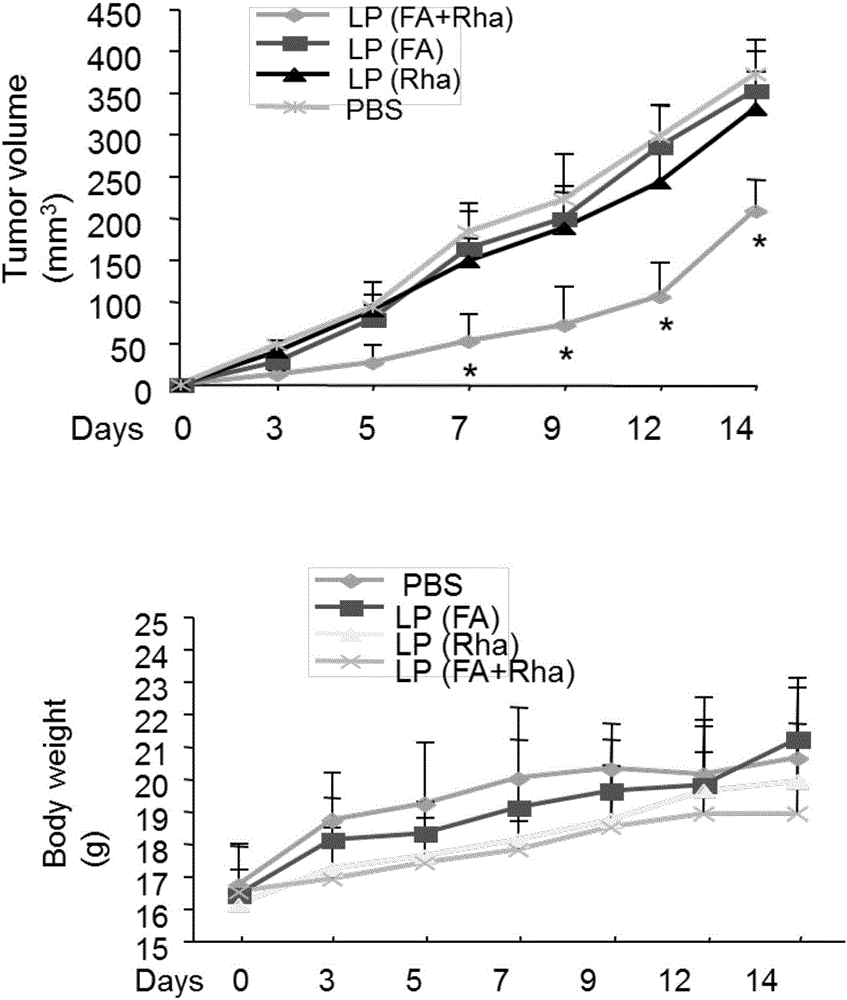
Liposome for cancer targeting immunotherapy by natural sugar antibody and preparation method of liposome
A technology of liposomes and phospholipids, which is applied in the direction of antineoplastic drugs, medical preparations of non-active ingredients, and pharmaceutical formulas, and can solve the problems of limiting the application of liposome-encapsulated drugs, large toxic and side effects, and easy leakage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Rhamnose-coupled polyethylene glycol distearate phosphate ethanolamine
[0098] Accurately weigh 40mg RhaNBuacid, dissolve it in 0.78ml dimethyl sulfoxide (DMSO), and then add 27mg 1,2 distearic acid-3 phosphatidylethanolamine-polyethylene glycol-amino (DSPE-PEG2000- NH2) and 0.39ml pyridine (pyridine), then add 81mg dicyclohexylcarbodiimide, react at room temperature for 4 hours. After forming a mist mixture, pyridine was removed by rotary evaporation, and 10 ml of single distilled water was added. The supernatant was collected by centrifugation, and the supernatant was dialyzed twice with 3000 molecular weight dialysis membrane, 50mM 2L saline solution, and 2L deionized water three times, and finally DSPE-PEG2000-Rhamnose was obtained as a white solid by lyophilizing the dialyzate. Synthetic route such as Figure 2-2 shown.
Embodiment 2
[0099] Example 2: Polyethylene glycol distearate phosphoethanolamine coupled with folic acid
[0100] Accurately weigh 79mg of folic acid, dissolve it in 2ml of dimethyl sulfoxide (DMSO), and then add 50mg of 1,2 distearic acid-3 phosphatidylethanolamine-polyethylene glycol-amino (DSPE-PEG2000-NH2 ) and 1ml of pyridine (pyridine), followed by adding 96mg of dicyclohexylcarbodiimide and reacting at room temperature for 4 hours. After forming a mist mixture, pyridine was removed by rotary evaporation, and 10 ml of single distilled water was added. The supernatant was collected by centrifugation, and the supernatant was dialyzed twice with 50mM 2L saline solution and three times with 2L deionized water on a dialysis membrane with a molecular weight of 1000. Finally, DSPE-PEG2000-Folate, a yellow solid, was obtained by freeze-drying the dialyzate. Synthetic route such as Figure 2-1 shown.
Embodiment 3
[0101] Example 3: Preparation of immune targeting liposomes
[0102] Preparation of 1,2-dioleoyl-SN-glycerol-3-phosphatidylethanolamine (DOPE), the rhamnose-coupled polyethylene glycol distearate phosphate ethanolamine (DSPE-PEG2000-Rhamnose) obtained in Example 1 , The folic acid-coupled polyethylene glycol distearic acid phosphodylethanolamine (DSPE-PEG2000-Folate) mother solution obtained in Example 2 with three component concentrations of 0.1M, 0.01M and 0.001M were stored at -80°C.
[0103]Take 20μl DOPE, 200μL DSPE-PEG2000-Rhamnose and 2μl DSPE-PEG2000-Folate to make a mixture with a molar ratio of 1000:1000:1 (diluted in 10ml chloroform / methanol (9 / 1)), and place in 250ml clean In a round bottom flask, spin dry on a rotary evaporator at 60 rpm. During rotary evaporation, the round-bottomed flask should be immersed in a 40°C water bath and spin-dried until a uniform lipid film appears. After 15 minutes, the round-bottomed flask left the water surface, and after passing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com