Pluripotent human stem cell technology, method and product of small molecule oriented tissue and organ regeneration
A technology of stem cells and human cells, applied in the field of biomedicine, can solve problems such as laborious purification or separation steps, unsuitable for commercial and clinical applications, unsuitable for clinical applications or human trials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] This example provides a method to induce high-quality clinical-grade xeno-free pluripotent human stem cells that are mass-manufactured (cGMP) by small molecule directed differentiation into functional human nerves or cells that can be used for tissue engineering, Drug development, drug testing, and cell therapy.
[0123] Undifferentiated pluripotent human embryonic stem cells were seeded and maintained in a defined culture system containing DMEM / F-12 or KO-DMEM (knockout-DMEM) (80%), knockout serum replacement (KO) (20%) , L-alanyl-L-glutamine or L-GLN (2mM), MEM non-essential amino acids (MNAA, 1X), mercaptoethanol (0.1mM), purified human laminin (40 μg / ml) [ Dilute 1:30 in cold DMEM / F-12] or laminin / collagen as matrix protein, and bFGF (basic fibroblast growth factor, 20 nanograms / milliliter [ng / ml]). Knockout serum substitutes (KO) can be replaced with defined human elements containing MEM essential amino acids (MEAA, 1X), human insulin (20 μg / ml) and ascorbic acid ...
Embodiment 2
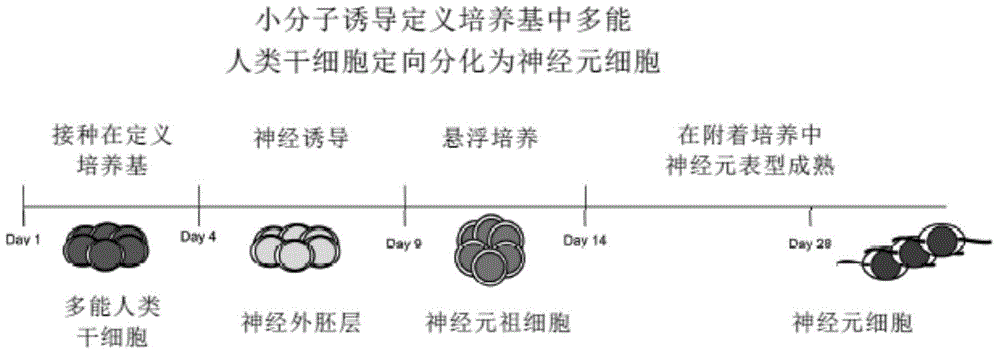
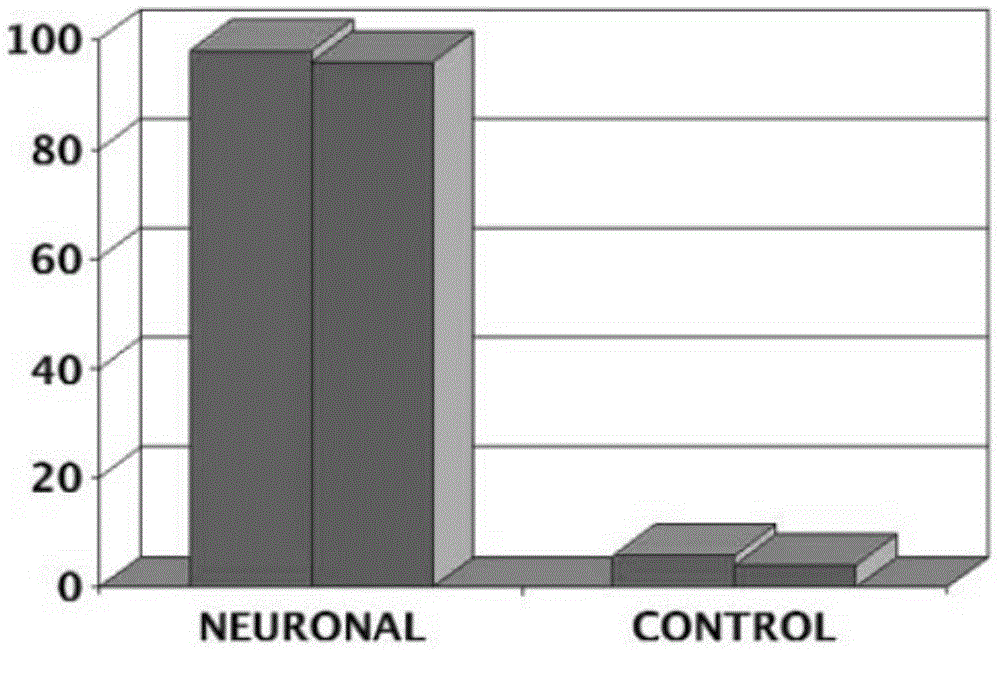
[0125] This example provides a method for the maintenance of high-quality clinical-grade xeno-free direct from large-scale manufacturing (cGMP) using current good manufacturing practice (cGMP) in a defined culture system through the use of retinoic acid (RA)-induced Directed and efficient differentiation of human embryonic stem cells into neuroectoderm by promoting the nuclear translocation of the neuron-specific transcription factor Nurr-1, and triggering the high-efficiency, high-purity, and large-scale development of lineage-specific neurons into human neural precursor cells and human Neuronal cells (>90%) ( figure 1 ,3,5).
[0126] Neuronal lineage-specific differentiation directly from the pluripotent state of human embryonic stem cells. Inoculate and maintain undifferentiated high-quality clinical-grade xeno-free pluripotent human embryonic stem cells in a defined culture system for 3 days, add RA (0.01mM) and continue to culture for 4-5 days under defined culture condi...
Embodiment 3
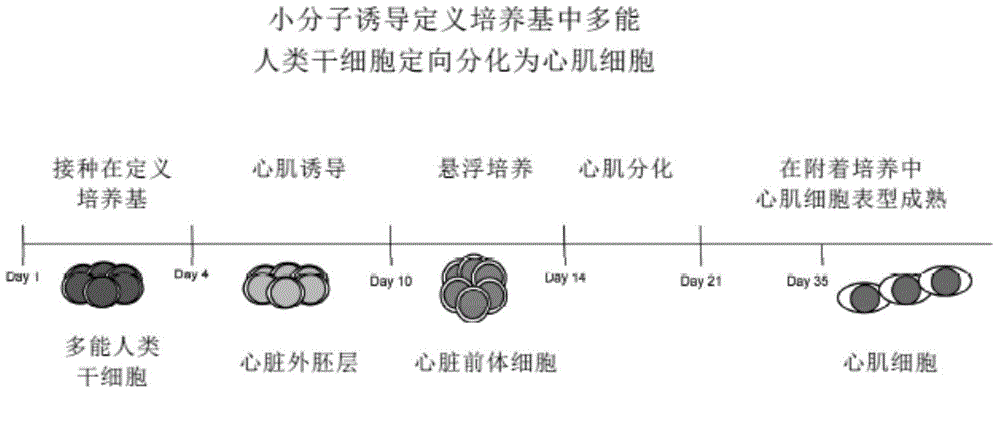
[0130] This example provides a method to induce xeno-free human cultures maintained in a defined culture system directly from high-quality clinical-grade mass-manufactured (cGMP) using current good manufacturing practice (cGMP) using nicotinamide (NAM). Directed and efficient differentiation of cardiac mesoderm from embryonic stem cells promotes the expression of the early cardiomyocyte-specific transcription factor CSX / Nkx2.5 and triggers the efficient, high-purity, and large-scale development of cardiac lineage-specific human cardiac precursors and beating human myocardium cells (>90%) ( figure 2 ,4,6).
[0131]Cardiomyocyte lineage-specific differentiation of pluripotent human embryonic stem cells using small molecules. Inoculate and maintain undifferentiated high-quality clinical-grade xeno-free pluripotent human embryonic stem cells in a defined culture system for 3 days, add NAM (10mM) and continue to culture for 4-5 days under defined culture conditions. These cardia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com