Method for expressing protein in resting spores of rhizopus stolonifer
A technology of Rhizopus glucoides and dormant spores, which is applied in the field of expressing proteins in dormant spores of Rhizopus glucoides , can solve problems such as inapplicability, and achieve excellent results with simple and fast steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Expression of green fluorescent protein (GFP) in Rhizopus glucoides cells:
[0067] 1. Plasmid construction
[0068] Using the GFP gene coding sequence, the GFP gene is shown in SEQ ID NO.5,
[0069] The protein sequence of the above GFP gene is shown in SEQ ID NO.6,
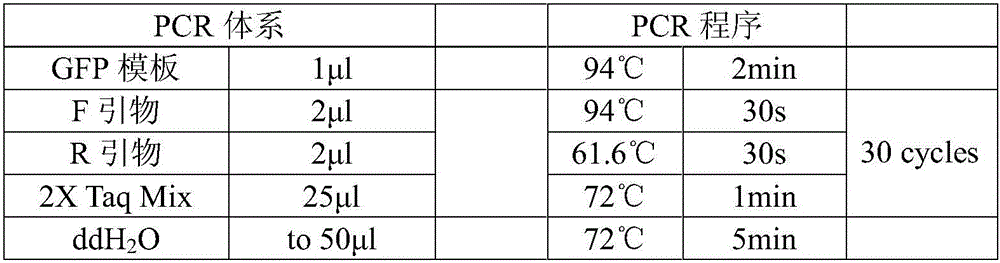
[0070] PCR primers for amplifying the GFP gene:
[0071] F: The wavy line is the Aat II restriction site
[0072] R: The wavy line is the Sal I restriction site
[0073] The underlined GCTAGC sequence in primer F is used to promote the binding of the translation initiation factor to RNA after the DNA is transcribed into RNA, and this sequence is adjacent to the initiation codon ATG of the expressed target gene. After using the above pair of primers for PCR, GCTAGC can be carried upstream of the GFP gene, and restriction sites can be carried upstream and downstream.
[0074]
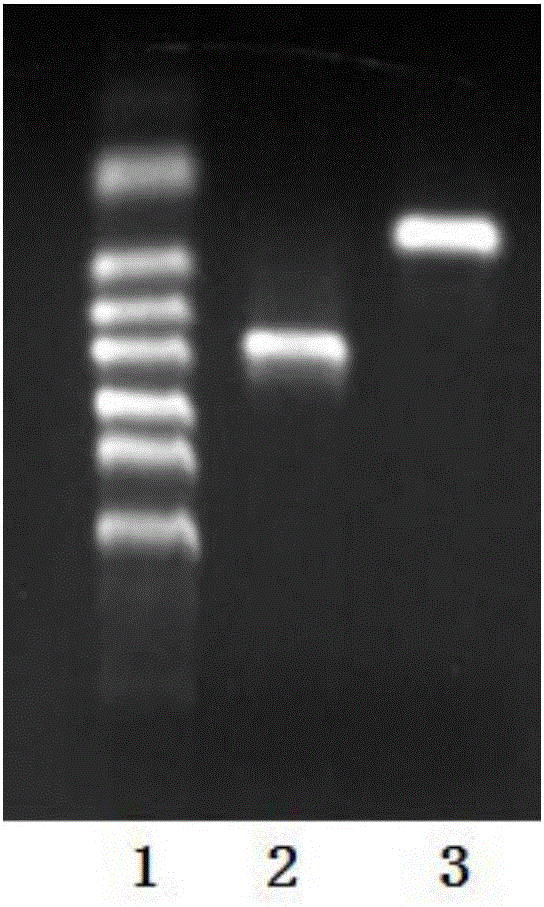
[0075] After agarose gel electrophoresis was used to detect that the PCR product was correct, the PCR product was recov...
Embodiment 2
[0105] Expression of red fluorescent protein (RFP) in Rhizopus glucoides cells:
[0106] 1. Plasmid construction
[0107] The nucleic acid sequence of RFP is shown in SEQ ID NO.7,
[0108] The protein sequence of RFP is shown in SEQ ID NO.8,
[0109] The primers for amplifying the RFP gene are as follows:
[0110] Upstream primer: RFP-F:5'CGGAATTC GCCACC ATGGCCTCCTCCGAGGACGT 3'
[0111] Downstream primer: RFP-R: 5' TCGAGCTCGTTAGGCGCCGGTGGAGTGG 3'
[0112] The 5-end of the upstream primer has an EcoR I restriction site and an underlined GCCACC sequence, which is used to promote the transcription of the DNA into RNA, and the translation initiation factor binds to the RNA. This sequence is consistent with the start codon of the expressed target gene ATG is next door.
[0113] The 5-end of the downstream primer has a Sal I restriction site.
[0114] According to the method of Example 1, the RFP gene was amplified by PCR, molecularly cloned, connected to the pGEM-T easy vect...
Embodiment 3
[0130] Expression of yellow fluorescent protein (YFP) in Rhizopus glucoides cells:
[0131] 1. Plasmid construction
[0132] The nucleic acid sequence of YFP is shown in SEQ ID NO.9,
[0133] The protein sequence of YFP is shown in SEQ ID NO.10,
[0134] Using the same primers as GFP in Example 1, and according to the same experimental steps and methods, the GFP gene was amplified by PCR, molecularly cloned, connected to the pGEM-T easy vector, and then transcribed in vitro, and the resulting RNA was transformed Host spores.
[0135] 2. Using the coding RNA of yellow fluorescent protein to express yellow fluorescent protein in the dormant spores of Rhizopus gluconeoides, the steps are as follows:
[0136] 1) Cultivation of Rhizopus glucoides and collection of spores
[0137] In a 15cm petri dish, prepare a solid agar medium (PDA medium), inoculate Rhizopus glucoides CICC 40325 on the surface of the solid agar medium, and cultivate it for 3 days at a temperature of 40°C and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com