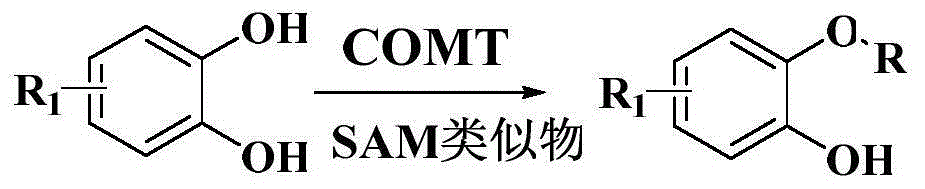
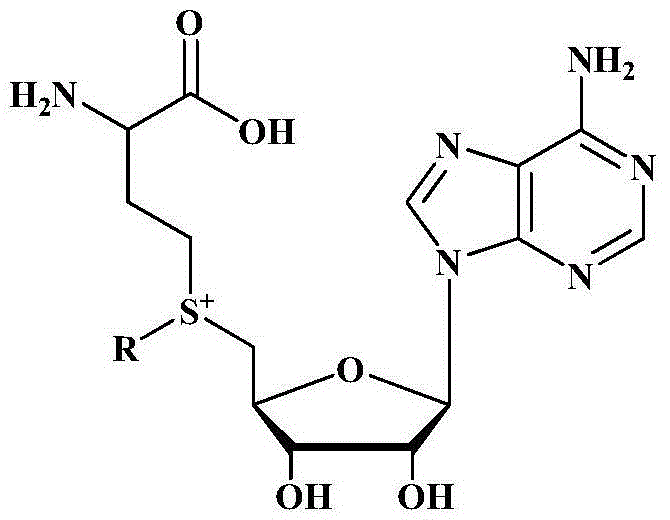
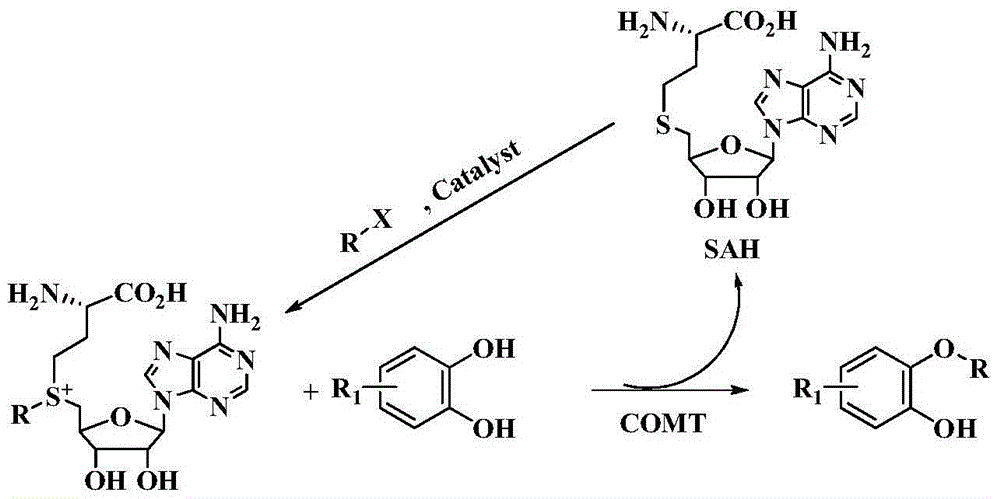
Method for biologically catalyzing hydrocarbylation of catechols
A technology of biocatalysis and biocatalyst, applied in the field of biocatalytic transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The purified COMT (final concentration 6.3mg / mL), Allyl-SAM (final concentration 4.0mM), catechol (final concentration 4.0mM) in 30mL containing 50mM Tris-Cl, pH 8.0, 1mM MgCl 2 , in a solution of 0.5mM EDTA, reacted at 40°C for 1h. Add 30mL of NaCl with a mass concentration of 26% to terminate the reaction, centrifuge to remove the precipitate, extract the supernatant ethyl acetate three times, remove the solvent by rotary evaporation of the organic phase, and separate the crude product through silica gel column chromatography, ethyl acetate / petroleum ether (1 / 10, v / v) was used as the eluent to obtain the target product 2-(allyloxy)phenol 1 with a yield of 48.3%.
[0049]
[0050] target product 1 1 H NMR (400MHz, CDCl 3 )δ4.61(d, J=5.2Hz, 2H), 5.42(dd, J=13.6, 36.6Hz, 2H), 5.64(s, 1H), 6.01-6.11(m, 2H), 6.79-6.94(m ,4H); 13 C NMR (160MHz, CDCl 3 )δ69.8, 112.2, 114.7, 118.2, 120.0, 121.7, 132.8, 145.5, 146.0; HRMS: Calculated for C 9 h 11 o 2 ([M+H] + ): 15...
Embodiment 2
[0052]The purified COMT (final concentration 8.5mg / mL), Allyl-SAM (final concentration 4.0mM), 2,3-dihydroxynaphthol (final concentration 6.0mM), allyl bromide (final concentration 4mM), Al 2 o 3 (0.4mM) Contains 60% 50mM NaH in 20mL 2 PO 4 , pH 5.5, 1 mM MgCl 2 , 1mM DTT, 40% methanol solution, stirred at 10°C for 72h. Add 20mL of 26% NaCl to terminate the reaction, centrifuge to remove the precipitate, extract the supernatant ethyl acetate three times, remove the solvent by rotary evaporation of the organic phase, separate the crude product through silica gel column chromatography, ethyl acetate / petroleum ether (1 / 10, v / v) was used as eluent to obtain the target product 2-(allyloxy)naphthol 2 with a yield of 80.2%.
[0053]
[0054] target product 2 1 H NMR (400MHz, CDCl 3 )δ4.75(dt,J 1 =5.6Hz,J 2 =1.2Hz,2H),5.35-5.50(m,2H),5.93(s,1H),6.09-6.18(m,1H),7.13(s,1H),7.27(s,1H),7.28-7.34( m,2H),7.66(dd,J=7.4,1.8Hz,2H); 13 CNMR (100MHz, CDCl 3 )δ69.7, 107.0, 109.5, 1...
Embodiment 3
[0056] The whole cell lysate (final concentration 5.8mg / mL) of Escherichia coli recombinant strain expressing COMT, Allyl-SAM (final concentration 4.0mM), dopamine (final concentration 4.0mM) in 30mL containing 50mM Tris-Cl, pH 8.5, 1mM DTT, 20mM MgCl 2 , in a solution of 10mM EDTA, after reacting at 37°C for 5h, add 30mL of 26% NaCl to terminate the reaction, centrifuge to remove the precipitate, extract the supernatant with ethyl acetate three times, remove the solvent by rotary evaporation of the organic phase, and separate the crude product by silica gel column chromatography. Methanol / dichloromethane (1 / 10, v / v) was used as the eluent to obtain the target product 3m with a yield of 20.1%.
[0057]
[0058] Target product 3m 1 H NMR (400MHz,D 2 O) δ3.15(t, J=7.4Hz, 2H), 4.55(t, J=6.0Hz, 2H), 5.21-5.34(m, 2H), 5.93-6.04(m, 1H), 6.72-6.93( m,3H); HRMS: Calculated for C 11 h 16 NO 2 ([M+H] + ):194.1181;obtained:194.1176;
[0059] HPLC measures the ratio of the two...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com