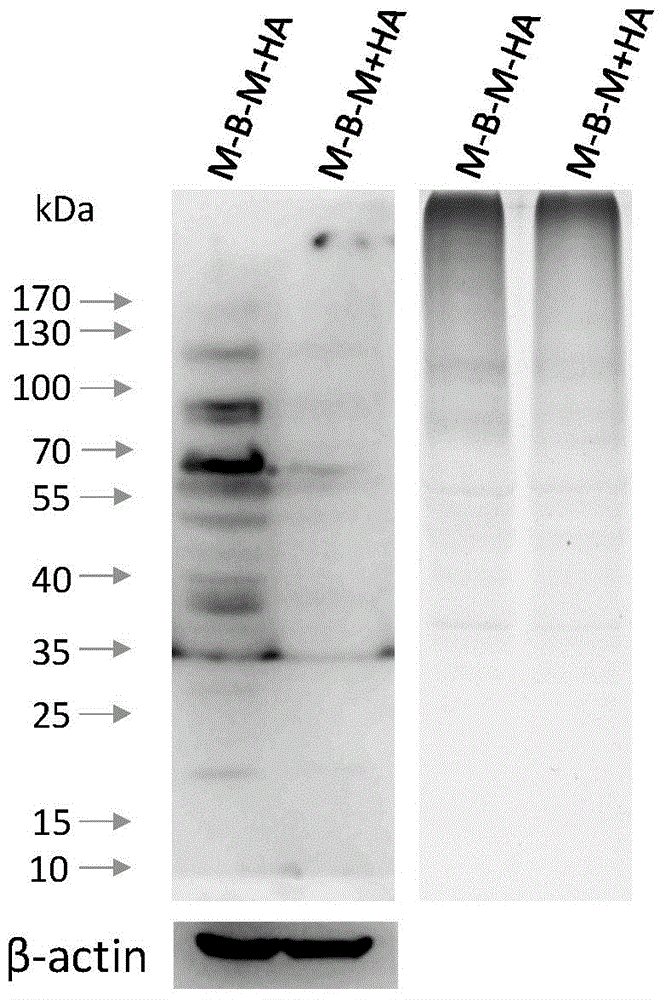
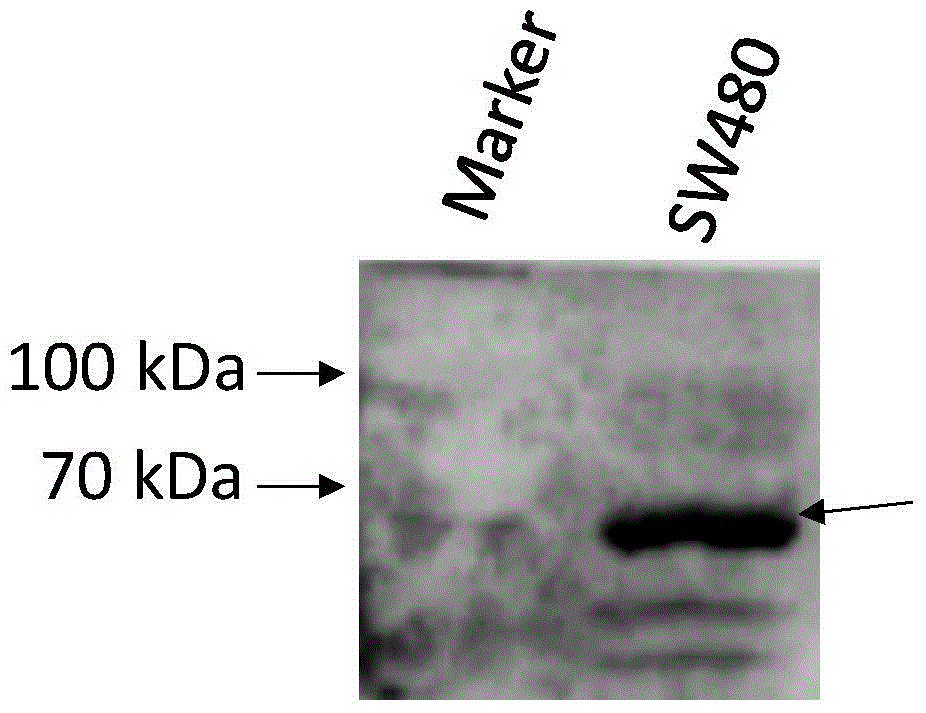
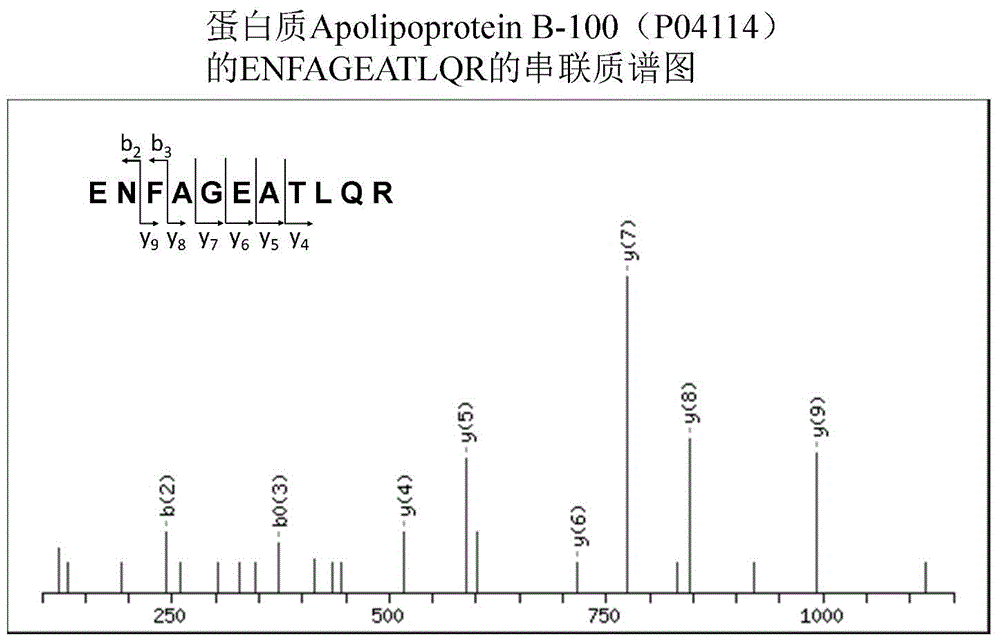
Test method of palmitoylated modified protein based on specific antibody
A palmitoylation and specific antibody technology, applied in the field of biochemical analysis, can solve the problems of lack of direct enrichment and identification, high false positive rate, complicated operation, etc., to achieve simple and easy operability, low false positive rate, specific strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of immune antigen and control antigen
[0029] The peptides containing palmitoylation modification and their control peptides were synthesized by methods known in the art, and the peptides were coupled to the carrier protein. In this example, Shanghai Ruixing Biotechnology Co., Ltd. completed the synthesis of the peptides Coupling with protein: Synthesize 5 mg of short peptide containing only two cysteines without palmitoylation modification (indicated by C-C) and 9 mg of short peptide containing only two cysteines with palmitoylation modification ( Denote by C-C(pal)), and couple C-C and C-C(pal) to bovine serum albumin BSA, denote as CC-BSA and C(pal)-BSA respectively, and couple C-C(pal) to mcKLH Above, represented by C(pal)-KLH.
Embodiment 2
[0030] Example 2 Preparation of protein-modified pan-antibody serum by antigen immunization
[0031] The prepared C(pal)-KLH immunization experiment rabbits were divided into three immunizations, the first immunization, 200ug / rabbit, the second and third immunization 100ug / rabbit, after the third immunization, routine carotid artery bloodletting, antiserum Store at -20°C after collection.
Embodiment 3
[0032] Example 3 Identification of Antiserum Specificity Using Competitive ELISA
[0033]1) Coating synthetic peptides and proteins C(pal)-KLH, C(pal)-BSA and CC-BSA as antigens at a concentration of 2 μg / ml, dilute the purified antiserum with blocking solution (2 times) (1: 100), carry out ELISA detection, compare the OD value after C(pal)-KLH, C(pal)-BSA and CC-BSA react with antiserum, judge the effect of antiserum, the result shows (as shown in Table 1) , when the antigen is the positive control mcKLH-C-C (pal), there is an obvious immune response; when BSA-C-C (pal) is used as the antigen, there is also a reaction, indicating that the anti-palmitoylation modified antiserum is effective, and its titer is relatively Low; 2) Reduce the dilution ratio of antiserum, dilute the purified antiserum (1:3 start) with blocking solution (2 times), and perform ELISA detection, compare C(pal)-KLH, C(pal) -BSA and CC-BSA reacted with the OD value of the antiserum to judge the effect of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com