Medicine combination for treating pericardial effusion and preventing complications
A technology for pericardial effusion and complications, which is applied in the field of pharmaceutical compositions for treating pericardial effusion and preventing complications, can solve the problems of unavoidable recurrence of complications, inaccurate disease control, increased risks, etc., so as to enhance human immunity The effect of improving anti-tumor and soothing emotions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
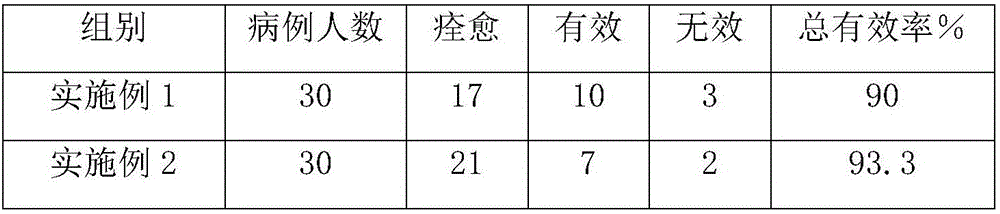
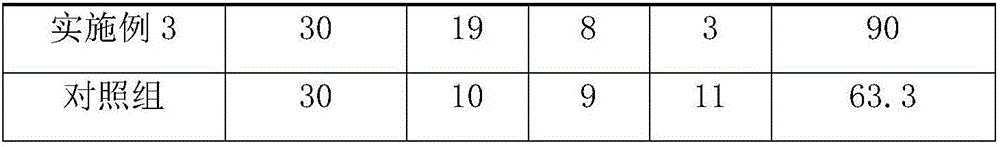
Embodiment 1
[0019] The pharmaceutical composition for treating pericardial effusion and preventing complications is mainly composed of the following ingredients in parts by weight: 0.4 parts of 4-pyridinecarbohydrazide, 0.1 parts of ofloxacin, 4.2 parts of apigenin, and 3.7 parts of hollynin 3.4 parts of ursolic acid, 3.1 parts of scopolamine, 2.6 parts of ergosterol, 2.3 parts of horse chestnut bark, 1.9 parts of oleanolic acid, 1.6 parts of oligosaccharides, 1.3 parts of oridonin, vitamin B2 0.005 parts, vitamin C 0.003 parts, low-substituted hydroxypropyl cellulose 10 parts.
[0020] Wherein, the said hollyside is Ilexin J, which is obtained by degrading the sugar chain of Ilexin O by alkali degradation method. Ilexin J has good antithrombotic pharmacological activity, and is of great importance to the research on the treatment of cardiovascular diseases. Significance, described alkali is the KOH solution that mass concentration is 7%; Described oligosaccharide comprises trehalose and ...
Embodiment 2
[0026] The pharmaceutical composition for treating pericardial effusion and preventing complications is mainly composed of the following ingredients in parts by weight: 0.95 parts of 4-pyridinecarbohydrazide, 0.4 parts of ofloxacin, 6.4 parts of apigenin, and 5.9 parts of hollynin 5.5 parts of ursolic acid, 5.15 parts of scopoletin, 4.6 parts of ergosterol, 4.2 parts of horse chestnut bark, 3.8 parts of oleanolic acid, 3.4 parts of oligosaccharides, 3 parts of oridonin, vitamin B2 0.011 parts, vitamin C 0.007 parts, low-substituted hydroxypropyl cellulose 17.5 parts.
[0027] Wherein, the said hollyside is Ilexin J, which is obtained by degrading the sugar chain of Ilexin O by alkali degradation method. Ilexin J has good antithrombotic pharmacological activity, and is of great importance to the research on the treatment of cardiovascular diseases. Significance, described alkali is the KOH solution that mass concentration is 7%; Described oligosaccharide comprises trehalose and...
Embodiment 3
[0033] The pharmaceutical composition for treating pericardial effusion and preventing complications is mainly composed of the following ingredients in parts by weight: 1.5 parts of 4-pyridine carbohydrazide, 0.7 parts of ofloxacin, 8.6 parts of apigenin, and 8.1 parts of hollynin 7.6 parts of ursolic acid, 7.2 parts of scopolamine, 6.6 parts of ergosterol, 6.1 parts of horse chestnut bark, 5.7 parts of oleanolic acid, 5.2 parts of oligosaccharides, 4.7 parts of oridonin, vitamin B2 0.017 parts, vitamin C 0.011 parts, low-substituted hydroxypropyl cellulose 25 parts.
[0034] Wherein, the said hollyside is Ilexin J, which is obtained by degrading the sugar chain of Ilexin O by alkali degradation method. Ilexin J has good antithrombotic pharmacological activity, and is of great importance to the research on the treatment of cardiovascular diseases. Significance, described alkali is the KOH solution that mass concentration is 7%; Described oligosaccharide comprises trehalose and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com