New method for synthesis of alkenyl thiocyanate derivative
An alkenyl thiocyanate and derivative technology, applied in organic chemistry and other directions, can solve the problems of weak product configuration selectivity, poor functional group compatibility, poisoning of experimental operators, etc., and achieves good product configuration selectivity, Compatible and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 25ml reaction vessel equipped with a reflux condenser, add 0.2mmol of phenylacetylene bromide, 0.02mmol of silver acetate, 0.4mmol of potassium thiocyanate and 2ml of glacial acetic acid, stir and react at 100°C for 3 hours , stop heating and stirring, cool to room temperature, add aqueous sodium bicarbonate solution after filtration and extract three times with ethyl acetate, dry over magnesium sulfate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield was 84%.
[0038] The structural characterization data of the resulting product are as follows:
[0039] IR(KBr):3065,2924,2854,2357,2120,735,692cm -1 .
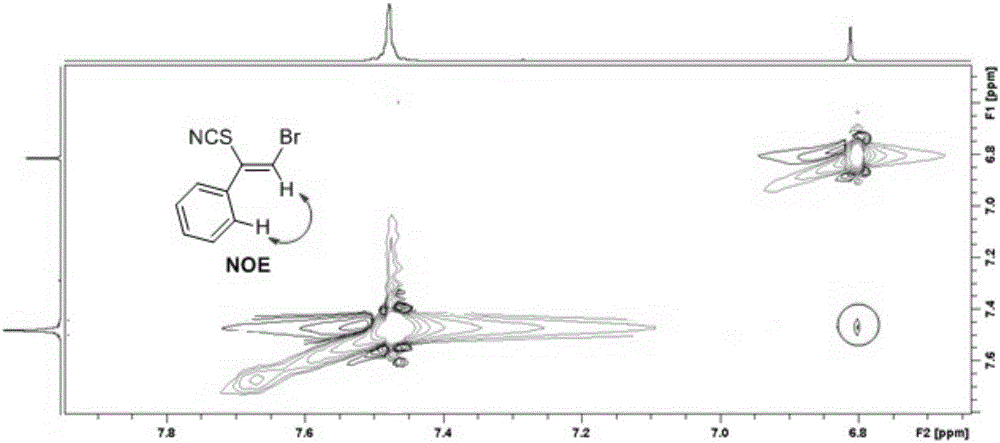
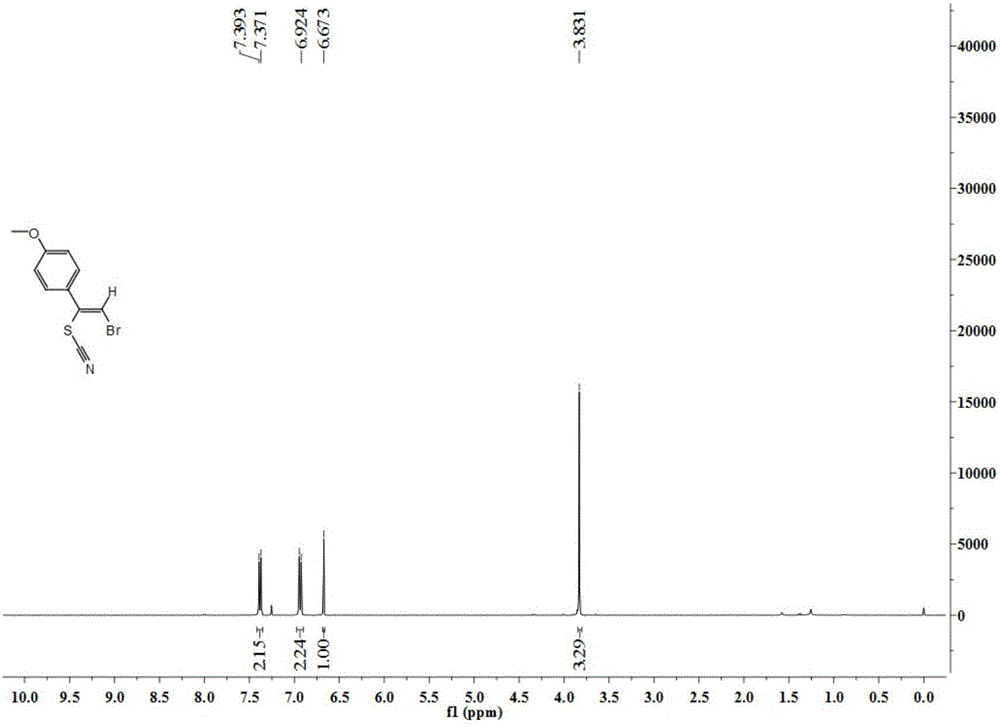
[0040] 1 H NMR (400MHz, CDCl 3 ):δ7.49-7.43(m,5H),6.79(s,1H).
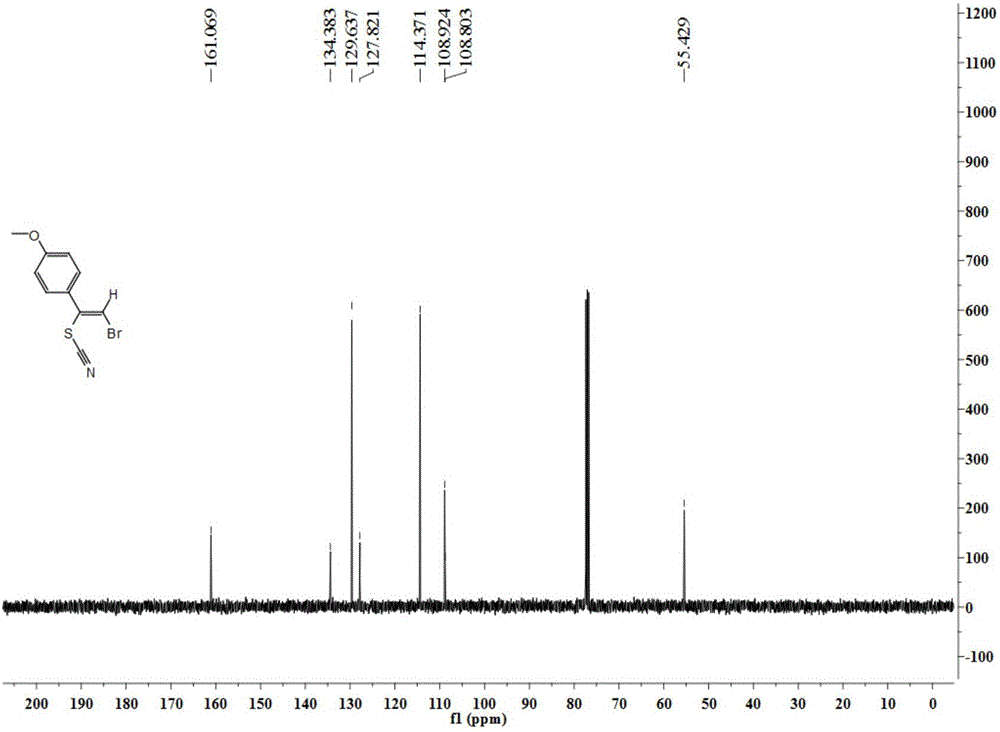
[0041] 13 C NMR (100MHz, CDCl 3 ): δ135.3, 134.4, 130.2, 128.9, 128.1, ...
Embodiment 2
[0045]In a 25ml reaction vessel equipped with a reflux condenser, add 0.2mmol of phenylacetylene bromide, 0.02mmol of silver chloride, 0.4mmol of potassium thiocyanate and 2ml of glacial acetic acid, and stir the reaction at 100°C for 3 hours After that, stop heating and stirring, cool to room temperature, add aqueous sodium bicarbonate solution after filtration and extract three times with ethyl acetate, dry over magnesium sulfate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent of the column chromatography was petroleum ether with a volume ratio of 100:1: ethyl acetate mixed solvent, and the yield was 56%.
Embodiment 3
[0047] In a 25ml reaction vessel equipped with a reflux condenser, add 0.2mmol of phenylacetylene bromide, 0.02mmol of silver bromide, 0.4mmol of potassium thiocyanate and 2ml of glacial acetic acid, and stir the reaction at 100°C for 3 hours After that, stop heating and stirring, cool to room temperature, add aqueous sodium bicarbonate solution after filtration and extract three times with ethyl acetate, dry over magnesium sulfate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent is petroleum ether: ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield is 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com