Anti-tumor macromolecule bonding drug with multidrug synergistic effect and preparation method thereof
A synergistic, anti-tumor drug technology, applied in the direction of anti-tumor drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor anti-cancer activity and low bioavailability of drugs, Achieve less side effects, inhibit tumor cell growth, and improve therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Preparation of polyethylene glycol monomethyl ether-b-polylactide-b-polyα-bromocaprolactone copolymer:
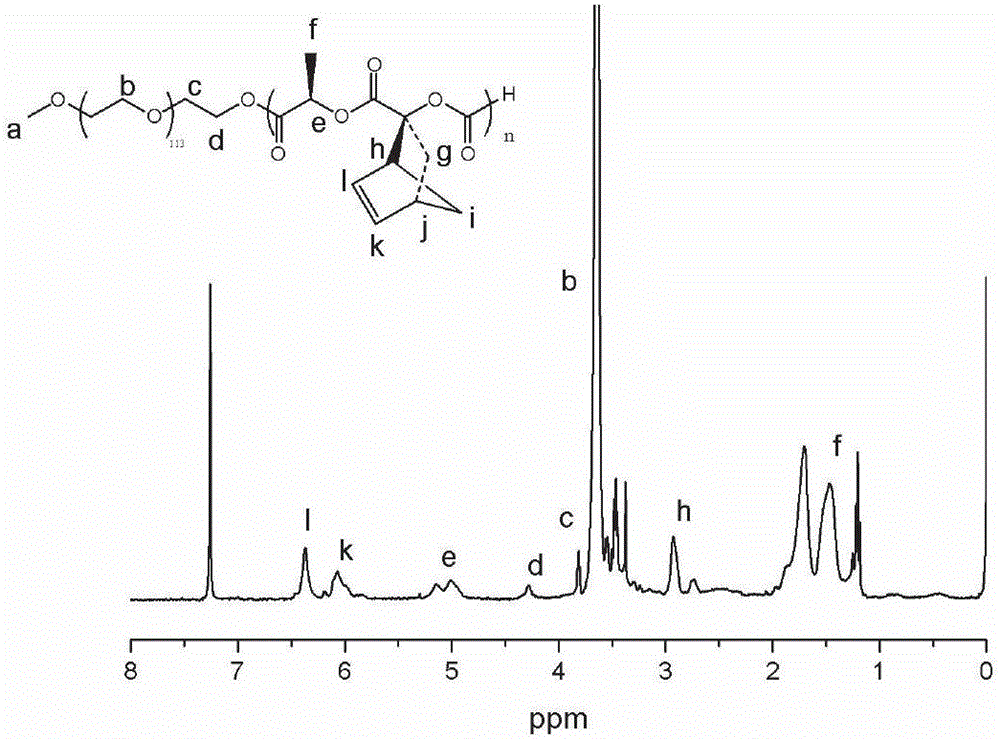
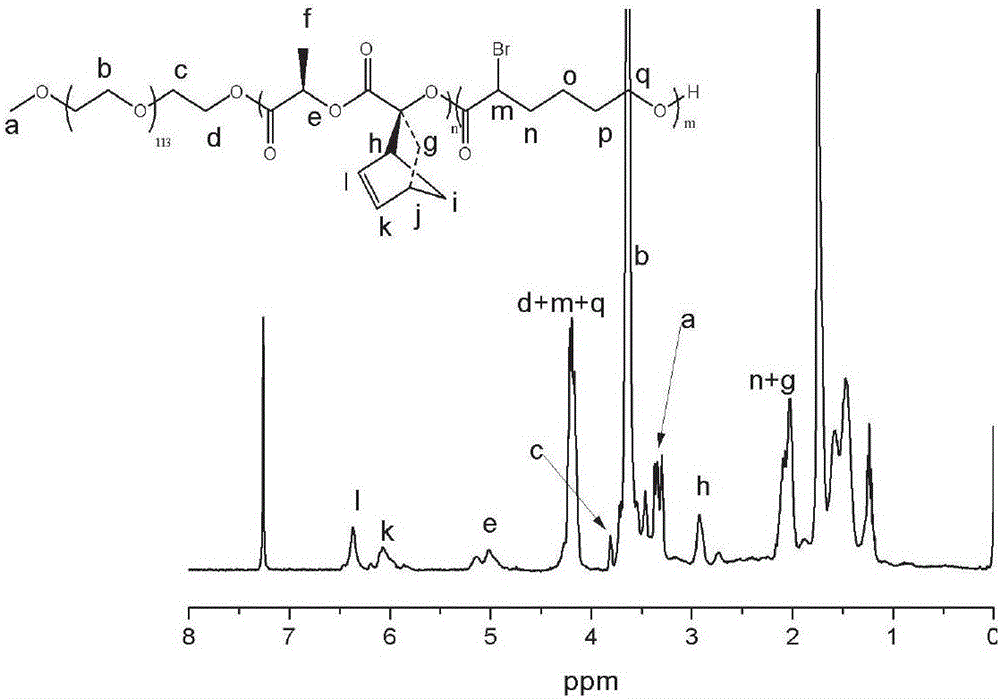
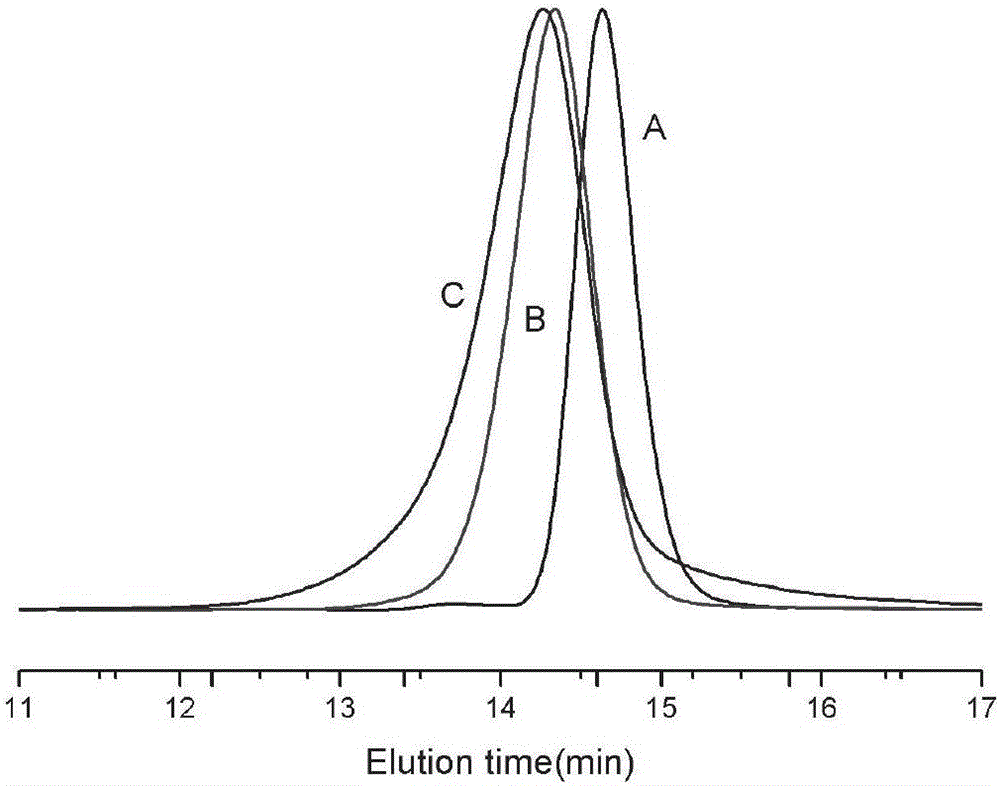
[0055] First, 0.80 g of lactide of norbornene pendant group and 1.00 g of polyethylene glycol monomethyl ether (mPEG) were added to an ampoule that had been vacuum-baked three times under nitrogen protection, and then 1 mL of purified DCM to make it completely dissolved, inject 1 mL of TBD solution containing initiator prepared from purified DCM, react at room temperature for 48 h, settle with anhydrous ether for 3 times, centrifuge and vacuum dry to obtain polyethylene glycol monomethyl ether-b- Polylactide diblock polymer, structurally characterized as figure 1 As shown, the molecular weight distribution is image 3 . Then, use it as a macromolecular initiator to initiate the polymerization of α-bromocaprolactone under the same polymerization conditions to obtain a polyethylene glycol monomethyl ether-b-polylactide-b-polyα-bromocaprolactone copolymer , whose ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com