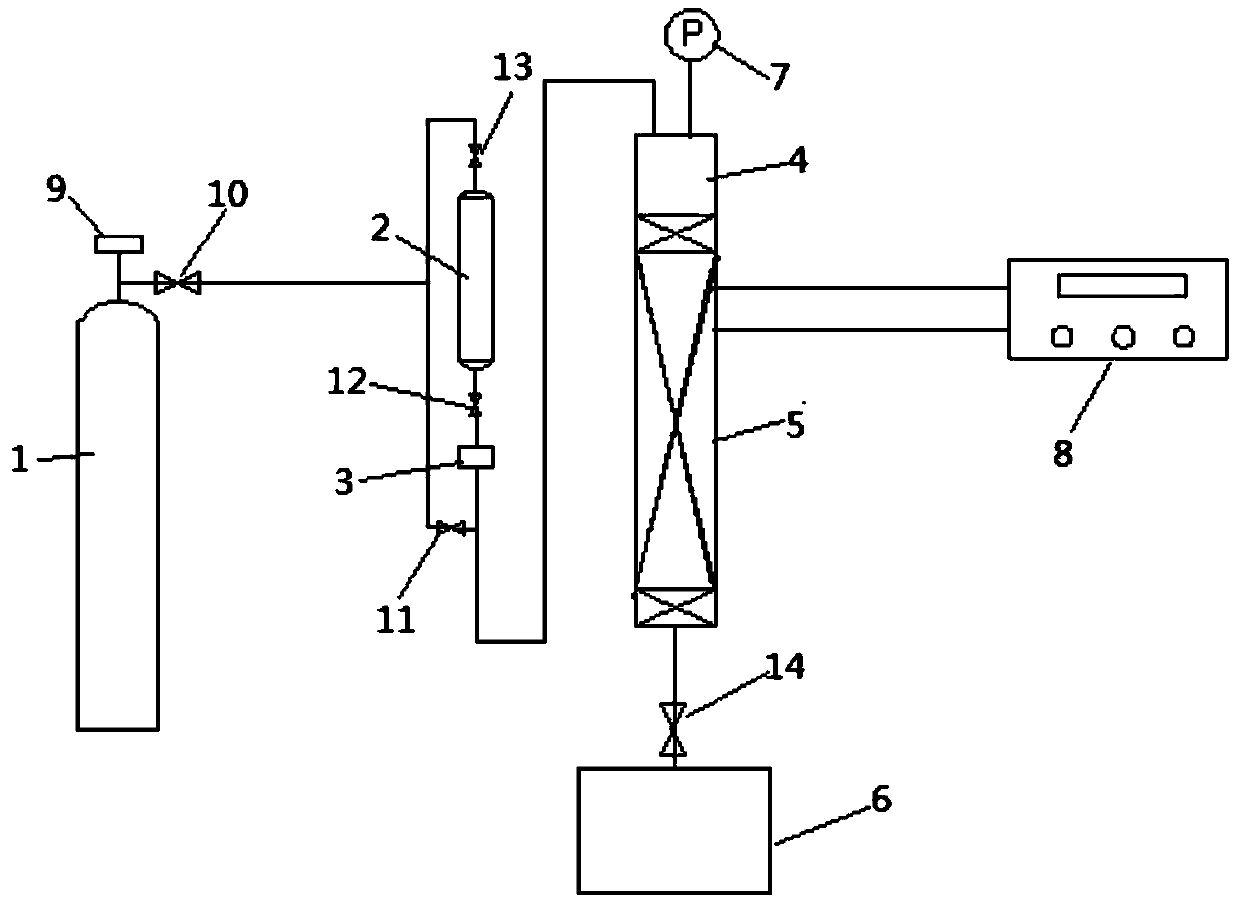
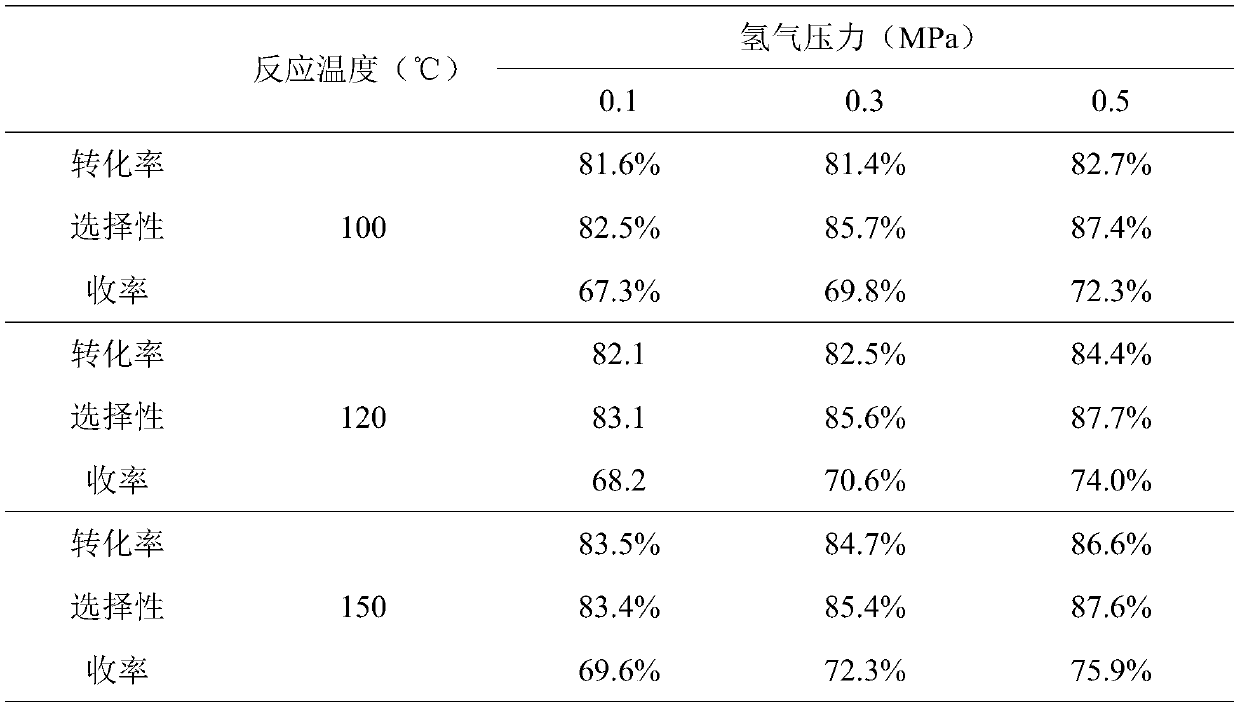
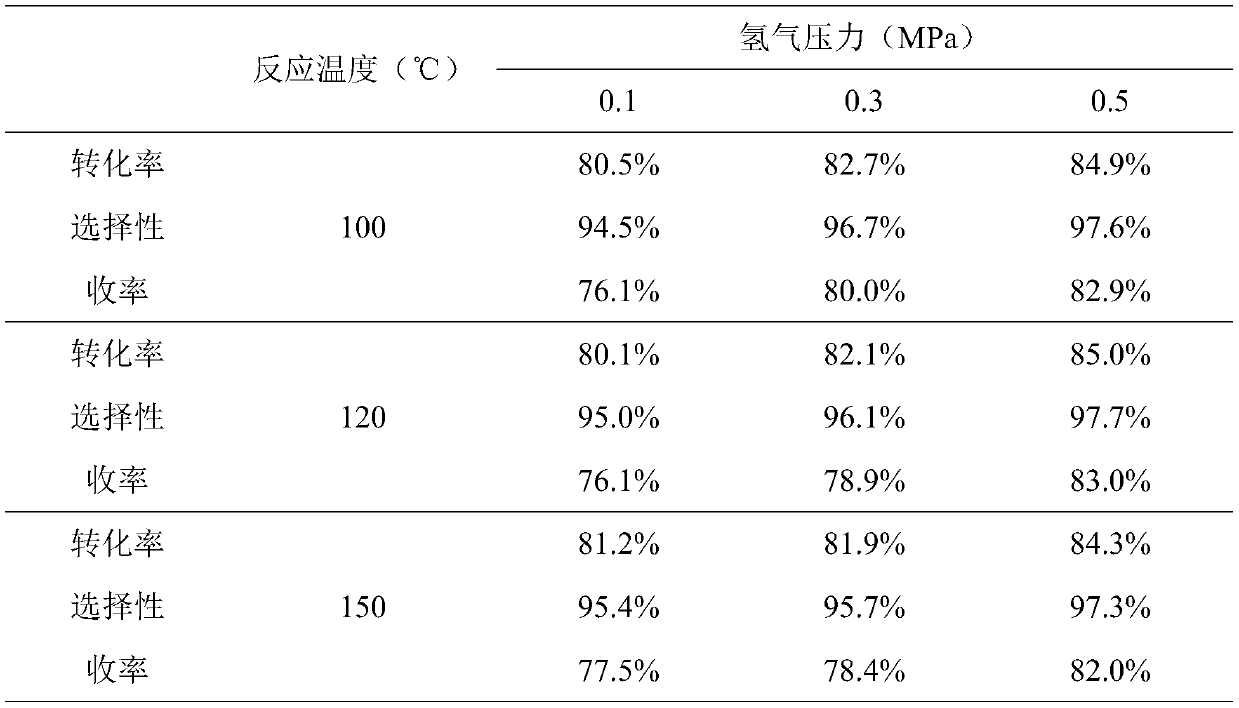
A fixed-bed continuous production device for preparing propionitrile and its application
A technology for a production device and a fixed bed reactor, which is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, organic chemistry, etc., can solve the problems of unsatisfactory conversion of acrylonitrile, harsh reaction conditions, and low selectivity of propionitrile, etc. Achieve the effect of inhibiting the disproportionation side reaction, low preparation cost and high selectivity of propionitrile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A catalyst for preparing propionitrile, the catalyst includes a carrier part and a metal salt part, the carrier part is gamma-alumina carrier, and the metal salt part includes nickel salt, copper salt and zinc salt.
[0032] The specific surface area of γ-alumina carrier is 220-225m 2 g -1 In the metal salt part, the weight percentage of nickel element accounts for 3%, the weight percentage of copper element accounts for 1%, and the weight percentage of zinc element accounts for 1%.
[0033]The catalyst has high activity, good stability, high dispersion of active components, long service life, high conversion rate of acrylonitrile, high selectivity of propionitrile, reducing the mass percentage of active component nickel makes the catalyst difficult to deactivate, and the addition of copper can Reduce the hydrogenation reaction activity, prevent the excessive hydrogenation of acrylonitrile to generate propylamine, and the addition of zinc can inhibit the occurrence o...
Embodiment 2
[0035] A catalyst for preparing propionitrile, the catalyst includes a carrier part and a metal salt part, the carrier part is gamma-alumina carrier, and the metal salt part includes nickel salt, copper salt and zinc salt.
[0036] The specific surface area of γ-alumina carrier is 220-225m 2 g -1 In the metal salt part, the weight percentage of nickel element accounts for 10%, the weight percentage of copper element accounts for 3%, and the weight percentage of zinc element accounts for 3%.
[0037] The catalyst has high activity, good stability, high dispersion of active components, long service life, high conversion rate of acrylonitrile, high selectivity of propionitrile, reducing the mass percentage of active component nickel makes the catalyst difficult to deactivate, and the addition of copper can Reduce the hydrogenation reaction activity, prevent the excessive hydrogenation of acrylonitrile to generate propylamine, and the addition of zinc can inhibit the occurrence...
Embodiment 3
[0039] A preparation method for a catalyst preparing propionitrile, characterized in that the steps are as follows:
[0040] Step 1. Carrier roasting: take the gamma-alumina carrier and place it in a muffle furnace, and roast it at 600-700°C for 6-8 hours to obtain the roasted carrier;
[0041] Step 2, metal salt loading, 31.29gNi(NO 3 ) 2 ·6H 2 O, 8.01gCu(NO 3 ) 2 ·3H 2 O and 9.58gZn(NO 3 ) 2 ·6H 2 O was dissolved in 200ml of water to obtain an impregnating solution, and then put into 200g of γ-alumina for thorough mixing and impregnation until the impregnating solution was completely absorbed by γ-alumina. Then it was dried at 120°C for 5 hours, then calcined at 650°C for 7 hours, and cooled down to room temperature naturally to obtain catalyst A.
[0042] Catalyst A contains 3% Ni, 1% Cu and 1% Zn by weight respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com