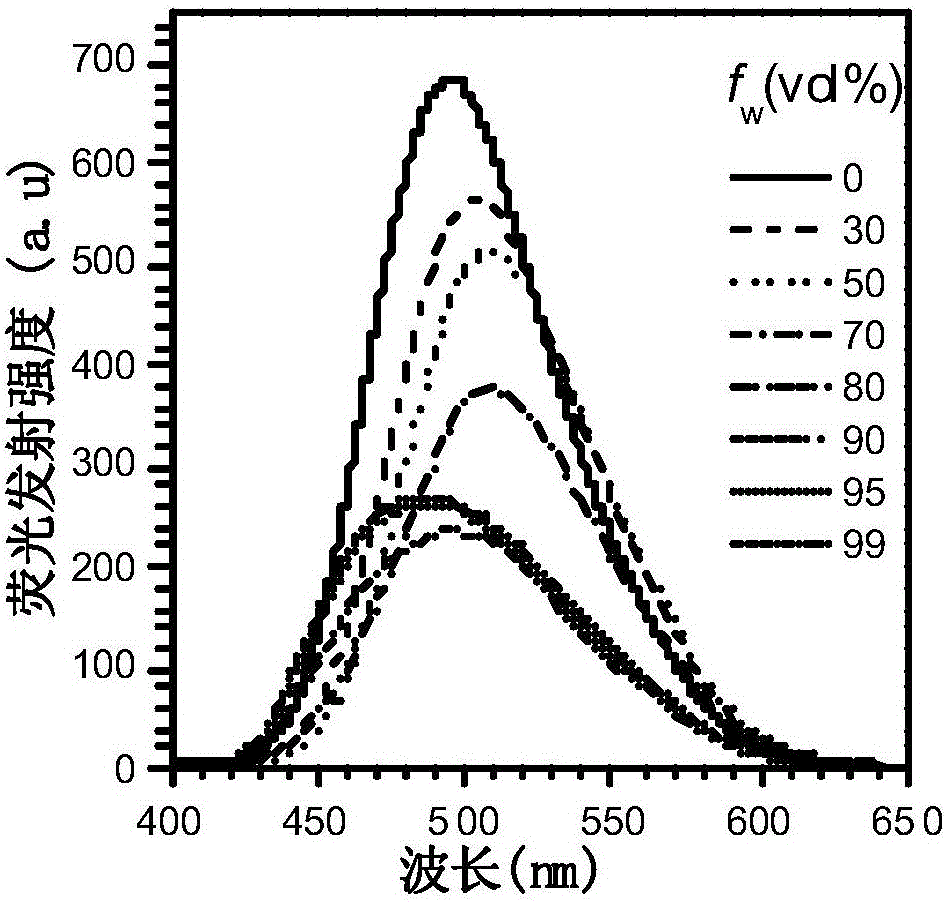
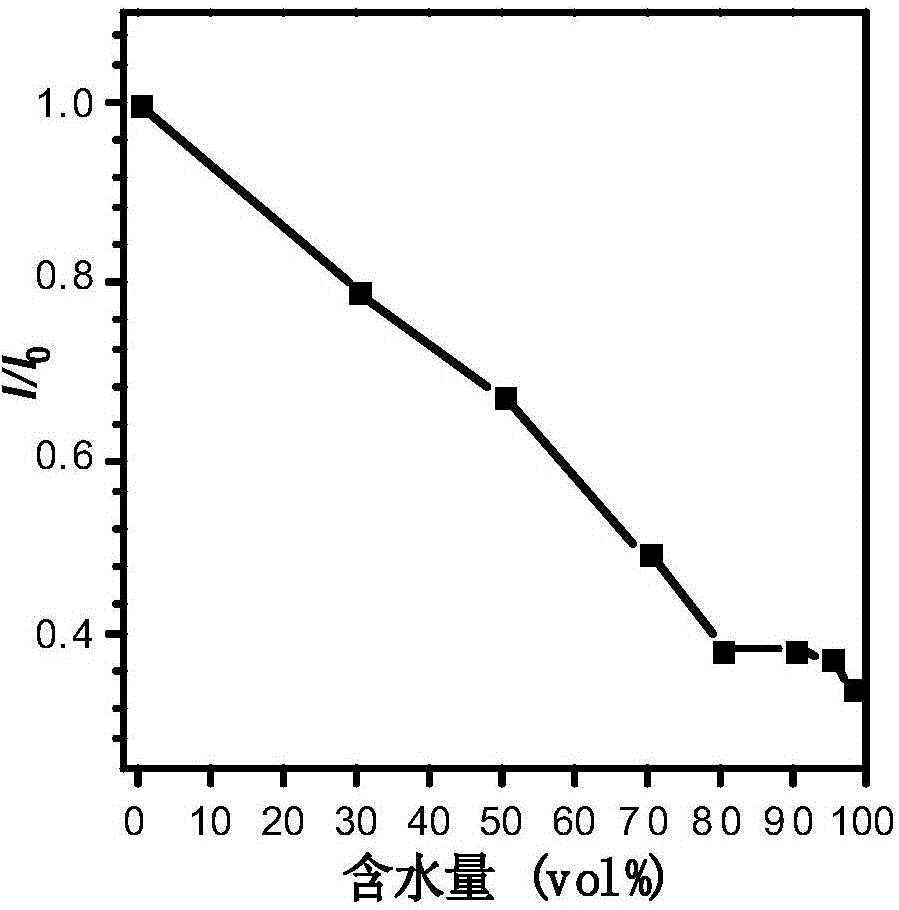
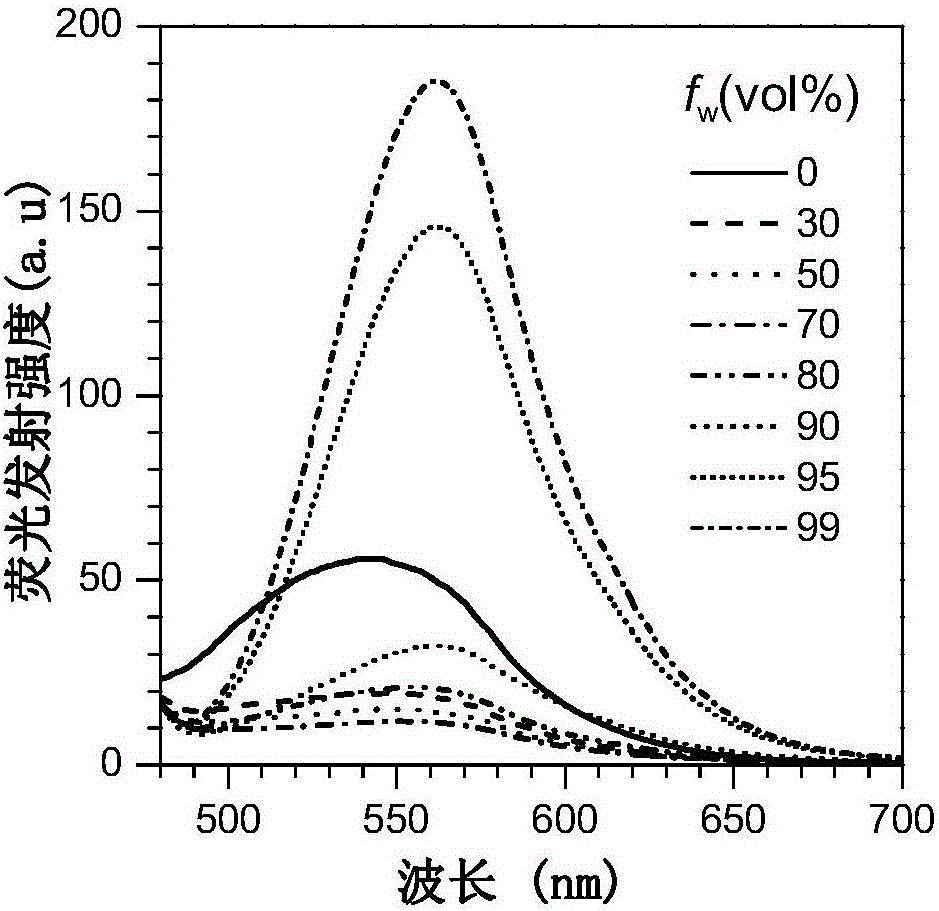
Fluorescent material with properties of aggregation-induced emission and piezochromism
A technology of aggregation-induced luminescence and fluorescent materials, applied in the field of fluorescent materials with aggregation-induced luminescence properties and piezochromic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of 1,4-diphenyl-2,2'-(1,4-phenylenebis(methylene))malononitrile with the following structural formula
[0043]
[0044] 1. Mix 0.0584g (0.2mmol) 2,5-dibromobenzene-1,4-dicarbaldehyde, 0.27mL DMF, 0.13mL 2mol / LNa 2 CO 3Aqueous solution, 0.0609g (0.5mmol) phenylboronic acid was added in 5mL round-bottomed flask, after stirring for 10 minutes, 0.0424g (0.01mmol) palladium acetate was added to the reaction solution, after the reaction was completed, extracted with ethyl acetate (3× 5mL), combined the organic phase, the organic phase was dried over anhydrous sodium sulfate, filtered, spin-dried, and the volume ratio of petroleum ether and ethyl acetate was 20:1 mixed liquid column chromatography to obtain 1,1':4', 1"-terphenyl-2',5'-dicarbaldehyde.
[0045] 2. Add 0.0400g (1.05mmol) 1,1':4',1"-terphenyl-2',5'-dicarbaldehyde to a 25mL round bottom flask, add absolute ethanol to dissolve it, and then add 0.1419 g (2.15mmol) malononitrile and 0.05mL 1mol / L NaOH ...
Embodiment 2
[0047] Synthesis of 1,4-bis(3-furyl)-2,2'-(1,4-phenylenebis(methylene))malononitrile with the following structural formula
[0048]
[0049] The phenylboronic acid in Example 1 was replaced with 3-furanboronic acid of the same amount, and the other steps were the same as in Example 1 to obtain 1,4-bis(3-furyl)-2,2'-(1,4 -Phenylene bis(methylene)) malononitrile, its productive rate is 63%, and the structural characterization data is: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 8.24 (s, 1H), 8.05 (s, 1H), 7.64 (t, 1H, J = 1.6Hz), 7.55 (s, 1H), 6.62-6.61 (q, 1H); 13 C-NMR (101MHz, CDCl 3 )δ (ppm): 157.36, 145.09, 142.34, 134.28, 132.85, 130.21, 122.40, 112.82, 111.99, 111.35, 87.65. HRMS (ESI-TOF) m / z: [M+Na] + Theoretical value C 22 h 10 N 4 o 2 Na, 385.0701, found 385.0703.
Embodiment 3
[0051] Synthesis of 1,4-bis(4-methoxyphenyl)-2,2'-(1,4-phenylenebis(methylene))malononitrile with the following structural formula
[0052]
[0053] The phenylboronic acid in Example 1 is replaced with 4-methoxyphenylboronic acid of the same amount of substance, and other steps are the same as in Example 1 to obtain 1,4-bis(4-methoxyphenyl)-2,2 '-(1,4-phenylene bis(methylene)) malononitrile, the yield is 69%, and the structural characterization data are: 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 8.22 (s, 1H), 7.84 (s, 1H), 7.27 (d, 2H, J = 8.5Hz, Ar H), 7.07 (d, 2H, J = 8.5Hz, Ar H), 3.91(s,3H,CH 3 ); 13 C-NMR (101MHz, CDCl 3 )δ (ppm): 160.97, 158.77, 142.91, 132.79, 131.50, 130.76, 128.94, 115.03, 113.09, 112.29, 86.53, 55.69. HRMS (ESI-TOF) m / z: [M+Na] + Theoretical value C 28 h 18 N 4 o 2 Na, 465.1327, found 465.1330.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com