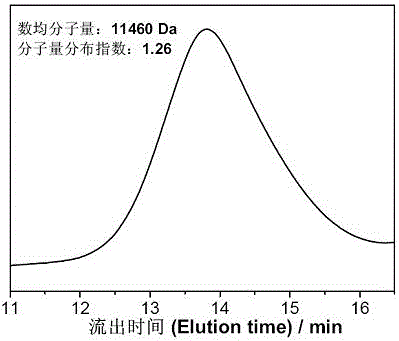
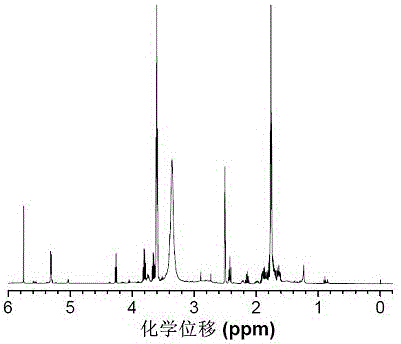
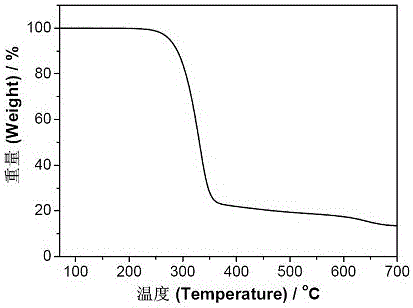
Preparation method of multi-sulfydryl hyperbranched polythioether based on alpha lipoic acid
A technology of lipoic acid and polysulfide, applied in the field of hyperbranched polymer preparation, achieving the effects of low cost, easy availability of raw materials, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add 1 part by weight of lipoic acid and 0.2 part by weight of propynyl alcohol to 20 parts by weight of tetrahydrofuran, stir for 5 minutes, then add 1.2 parts by weight of N,N'-dicyclohexylcarbodiimide React with 0.5 parts by weight of 4-dimethylaminopyridine at 25° C. for 12 hours to obtain a product containing both disulfide bonds and triple bonds.
[0019] (2) 1 weight part of the product containing disulfide bonds and triple bonds prepared in step (1) is dissolved in 100 parts by weight of tetrahydrofuran, and then 3.6 parts by weight of dithiothreitol and 100 parts by weight of water are added After reacting at 20°C for 8 hours, a product containing both sulfhydryl and triple bonds was obtained.
[0020] (3) Dissolve 1 part by weight of the product containing both mercapto groups and triple bonds prepared in step (2) in 3 parts by weight of N,N-dimethylformamide, and then add 0.02 parts by weight of 2-hydroxy- After 4'-(2-hydroxyethoxy)-2-methylpropiophenone,...
Embodiment 2
[0022] (1) Add 1 part by weight of lipoic acid and 0.1 part by weight of propynyl alcohol to 5 parts by weight of tetrahydrofuran, stir for 5 minutes, then add 1 part by weight of N,N'-dicyclohexylcarbodiimide After reacting with 0.2 parts by weight of 4-dimethylaminopyridine at 20° C. for 3 hours, a product containing both disulfide bonds and triple bonds was obtained.
[0023] (2) The product containing disulfide bond and triple bond that 1 weight part of step (1) is prepared is dissolved in 5 weight parts of tetrahydrofuran, then add 2 weight parts of dithiothreitol and 5 weight parts of water After reacting at 25°C for 24 hours, a product containing both sulfhydryl and triple bonds was obtained.
[0024] (3) Dissolve 1 part by weight of the product containing both mercapto groups and triple bonds prepared in step (2) in 0.5 parts by weight of N,N-dimethylformamide, and then add 0.03 parts by weight of 2-hydroxy- After 2-methyl-1-phenylacetone, it was irradiated under a 50...
Embodiment 3
[0026] (1) Add 1 part by weight of lipoic acid and 0.3 parts by weight of 3-butyn-1-ol to 100 parts by weight of tetrahydrofuran, stir for 5 minutes, then add 1.3 parts by weight of N,N'-bicyclo Hexylcarbodiimide and 0.45 parts by weight of 4-dimethylaminopyridine were reacted at 10° C. for 24 hours to obtain a product containing both disulfide bonds and triple bonds.
[0027] (2) 1 weight part of the product containing disulfide bonds and triple bonds prepared in step (1) is dissolved in 20 weight parts of tetrahydrofuran, and then 5 weight parts of cysteine and 40 weight parts of water are added After reacting at 10°C for 12 hours, a product containing both mercapto and triple bonds was obtained.
[0028] (3) Dissolve 1 part by weight of the product containing both mercapto groups and triple bonds prepared in step (2) in 1.5 parts by weight of N,N-dimethylformamide, and then add 0.02 parts by weight of 2-dimethylformamide After amino-2-benzyl-1-[4-(4-morpholinyl) phenyl]-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com