Primers, probe, method and kit for detecting mycoplasma pneumoniae nucleic acid and drug-resistant mutation through real-time fluorescent PCR
A Mycoplasma pneumoniae, real-time fluorescence technology, applied in the field of molecular biology, can solve the problems of Mycoplasma pneumoniae, such as long time-consuming, inaccurate detection results, harsh growth conditions, etc., and achieve high accuracy, high sensitivity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
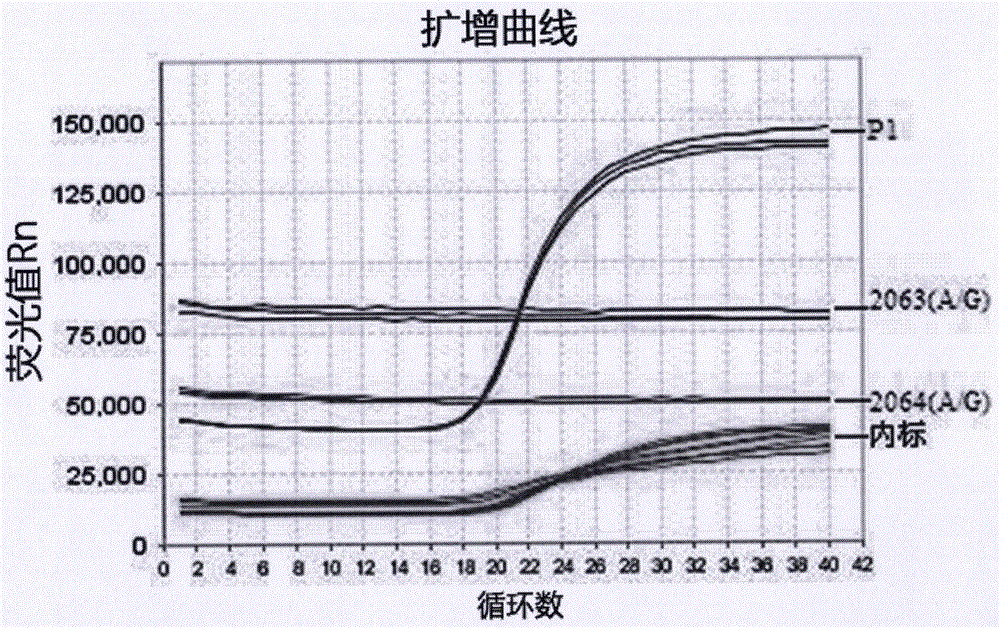
[0024] The embodiment of the present invention provides a primer and probe for real-time fluorescent PCR detection of Mycoplasma pneumoniae nucleic acid and drug resistance mutation, and the nucleotide sequence of the primer for detection of Mycoplasma pneumoniae-specific P1 gene is shown in SEQ ID NOs: 2-3, to detect Mycoplasma pneumoniae The nucleotide sequence of the Taqman probe specific for the P1 gene is shown in SEQ ID NO: 4; the nucleotide sequence of the primers for detecting the resistance mutation sites 2063 (A / G) and 2064 (A / G) genes is shown in SEQ ID NO: 6 ~7, the nucleotide sequence of the Taqman probe for detecting the 2063 (A / G) gene of the drug resistance mutation site is shown in SEQ ID NO: 8; the Taqman probe for detecting the 2064 (A / G) gene of the drug resistance mutation site The nucleotide sequence of the needle is shown in SEQ ID NO: 10; the nucleotide sequence of the primer for detecting the internal standard gene int is shown in SEQ ID NO: 12-13, and ...
Embodiment 2
[0044] The embodiment of the present invention also provides a real-time fluorescent PCR method for detecting Mycoplasma pneumoniae nucleic acid and drug resistance mutation, comprising:
[0045] Step 101, sample nucleic acid preparation to obtain a nucleic acid template;
[0046] Step 102: Design specific primers and fluorescently labeled probes for the Mycoplasma pneumoniae-specific P1 gene, 2063 (A / G) gene, 2064 (A / G) gene, and internal standard gene int, respectively;
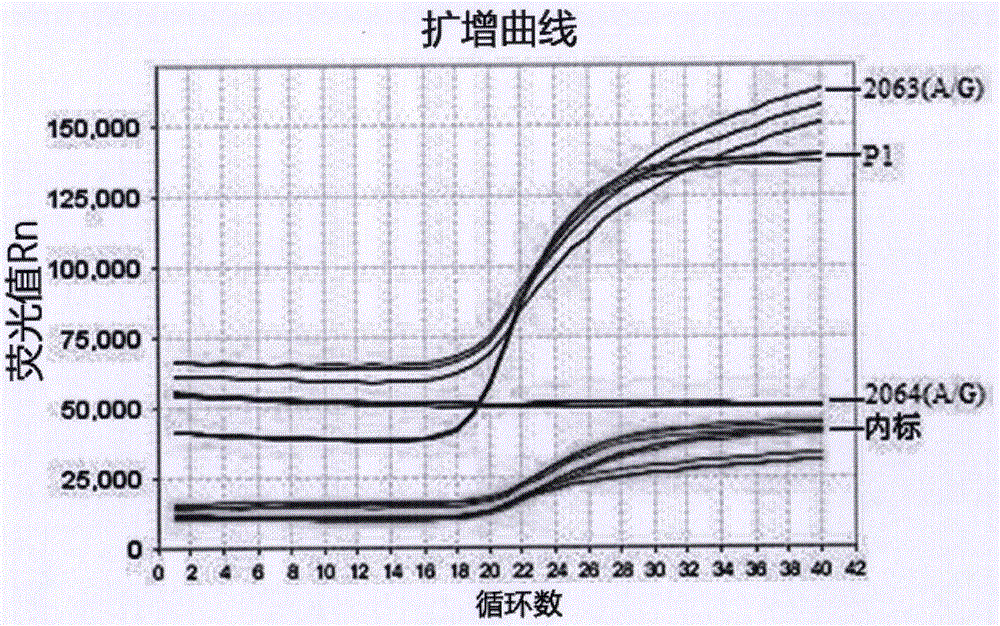
[0047] Among them, the nucleotide sequences of primers for detecting Mycoplasma pneumoniae-specific P1 gene are shown in SEQ ID NOs: 2-3, and the nucleotide sequences of Taqman probes for detecting Mycoplasma pneumoniae-specific P1 gene are shown in SEQ ID NO: 4; detection of drug resistance The nucleotide sequences of the primers of the mutation sites 2063 (A / G) and 2064 (A / G) genes are shown in SEQ ID NOs: 6-7, and the Taqman probes of the 2063 (A / G) gene at the drug resistance mutation site are detected....
Embodiment 3
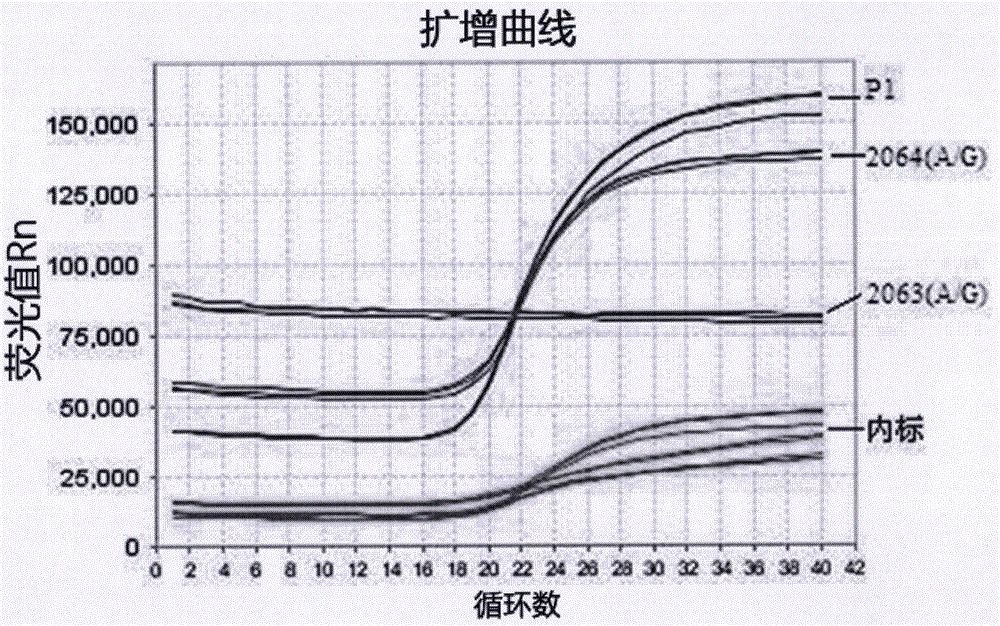
[0059] The embodiment of the present invention provides a kit for real-time fluorescent PCR detection of Mycoplasma pneumoniae nucleic acid and drug resistance mutation, including nucleic acid extraction solution, a first primer-probe mixture, a second primer-probe mixture, and a third primer-probe Mixed solution, PCR reaction enzyme system, internal standard, negative control substance, positive control substance, and packaging boxes for separating and centrally packaging these reagent bottles or tubes, wherein the first primer-probe mixture is composed of deoxyribonucleoside triphosphate dN(U)TP, the upstream and downstream primers of the P1 gene and a fluorescently labeled probe are composed of the upstream and downstream primers of the internal standard gene and a fluorescently labeled probe; the second primer-probe mixture is composed of deoxyribose nuclei The glucoside triphosphate dN(U)TP, the upstream and downstream primers of the 2063(A / G) gene and a fluorescently labe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com