Preparation method of iron-titanium oxide defluorination adsorbent
A technology of iron oxides and oxides, applied in chemical instruments and methods, adsorption of water/sewage treatment, water pollutants, etc., can solve the problems of acidic pH range, harmful ion dissolution, high cost of adsorbents, etc. Mild conditions, simple and easy-to-control preparation method, and high fluoride removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
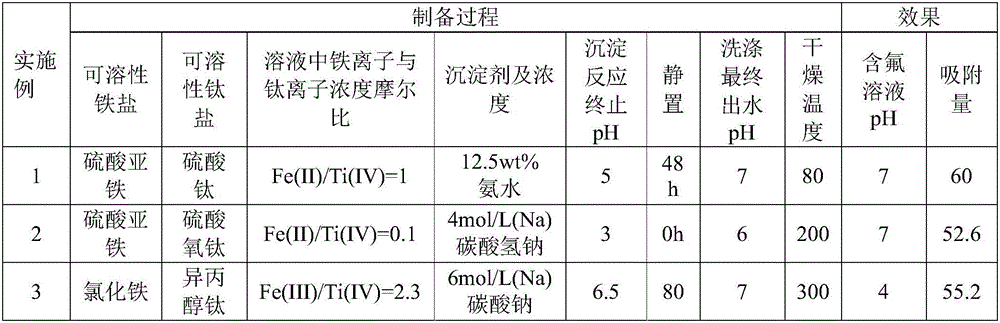
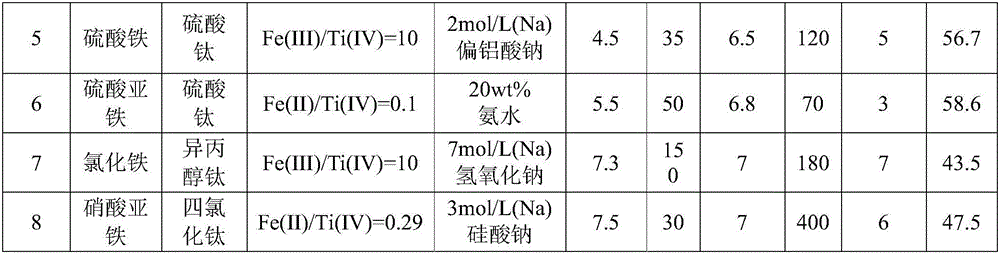
[0026] Example 1, preparation of iron-titanium oxide fluorine removal adsorbent
[0027] Prepare 200mL of ferrous sulfate and titanium sulfate mixed solution, wherein the iron ion concentration is 0.2mol / L, the titanium ion concentration is 0.2mol / L, under stirring state, add 12.5(wt)% ammonia water, when the pH value end point reaches Stop joining at 5 o'clock. After standing still for 48 hours, the precipitate generated by the reaction was washed with deionized water until the pH value of the effluent was 7, filtered, and dried at 80° C. to constant weight. The dried product was ground to obtain a fluorine-removing adsorbent. When the pH of the aqueous solution is 7, the saturated adsorption capacity of the adsorbent for fluorine in water reaches 60.0 mg / g.
Embodiment 2
[0028] Example 2, preparation of iron-titanium oxide defluorination adsorbent
[0029] Prepare 200 mL of mixed solution of ferrous nitrate and titanyl sulfate, in which the concentration of iron ions is 0.02 mol / L, and the concentration of titanium ions is 0.2 mol / L. Under stirring, add sodium bicarbonate solution with a concentration of sodium ions of 4 mol / L , the addition was stopped when the pH endpoint reached 3. After standing still for 0 h, the precipitate generated by the reaction was washed with deionized water until the pH of the effluent was 6, filtered, and dried at 200°C to constant weight, and the dried product was ground to obtain a fluorine-removing adsorbent. When the pH of the aqueous solution is 7, the saturated adsorption capacity of the adsorbent for fluorine in water reaches 52.6 mg / g.
Embodiment 3
[0030] Example 3, preparation of iron-titanium oxide defluorination adsorbent
[0031] Prepare 200mL of ferric chloride and titanium isopropoxide mixed solution, wherein the concentration of iron ion is 0.35mol / L, the concentration of titanium ion is 0.15mol / L, under stirring state, add sodium carbonate solution with a concentration of sodium ion of 6mol / L , the addition was stopped when the pH end point reached 6.5. After standing still for 80 hours, the precipitate generated by the reaction was washed with deionized water until the pH of the effluent was 7, filtered, and dried at 300° C. to constant weight. The dried product was ground to obtain a fluorine-removing adsorbent. When the pH of the aqueous solution is 4, the saturated adsorption capacity of the adsorbent for fluorine in water reaches 55.2 mg / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com