Monopterus albus gene editing method
A gene editing, eel technology, applied in the field of eel gene editing, can solve the problem of not establishing gene editing technology and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Obtaining fertilized embryos at the 1-cell stage of rice field eel:
[0032] Artificial oxytocin breeding of rice field eels was carried out in an indoor breeding system, and the oxytocin hormone was produced by Ningbo Sansheng Pharmaceutical Co., Ltd. The injection dose of female eel (50-100g) was injected with 3mg of carp pituitary gland (PG), 10μg of luteinizing hormone releasing hormone A2 (LHRH-A2) and 500IU of chorionic gonadotropin (HCG) per 100g of female eel body weight. ). 24 hours after female eel injection, male eel (>200g) was injected with LHRH-A2 at 5μg / 100g body weight. After 40 hours of female eel injection, the eggs were squeezed out; at the same time, male eel sperm were obtained, and the sperm were placed in Danielal buffer (1.74mol / L NaCl, 0.02mol / L KCl, 0.01mol / L MgSO 4 ·7H 2 O,0.02mol / LCa(NO 3 ) 2 4H 2 O, 0.15mol / L Hepes, pH 7.2) immediately poured into the eggs, shake gently to mix, and let stand for 5min to complete the fertilization proce...
Embodiment 2
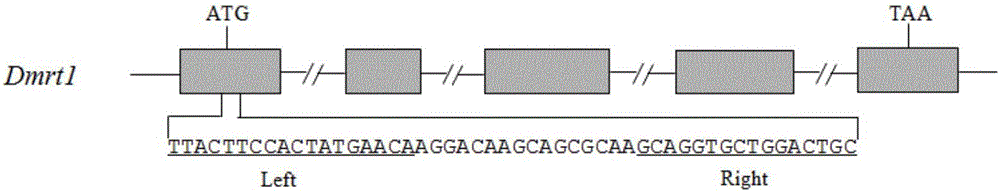
[0034] Construct the TALENs vector of the eel target gene (Dmrt1):
[0035] Taking the target gene Dmrt1 of rice field eel as an example, the TALENs target site design website (https: / / tale-nt.cac.cornell.edu / ) was used to select the "AGGACAAGCAGCGCAA" sequence of the Dmrt1 gene as the gene editing target. The recognition sequence of the arm is TTACTTCCACTATGAACA, and the recognition sequence of the right arm of TALENs is GCAGGTGCTGGACTGC( figure 1 ). According to the optimized "Golden Gate" cloning method reported in the literature, the TALENs expression vector targeting the rice field eel Dmrt1 gene was assembled and constructed (Liu et al., 2014). Plasmids for each repeat fragment (pNI1-pNI10 corresponds to bases A1-A10, pNN1-pNN10 corresponds to bases G1-G10, pHD1-pHD10 corresponds to bases C1-C10, pNG1-pNG10 corresponds to bases T1-T10) , and the plasmids corresponding to the last base (pLRNI corresponds to base A, pLRNN corresponds to base G, pLRHD corresponds to base ...
Embodiment 3
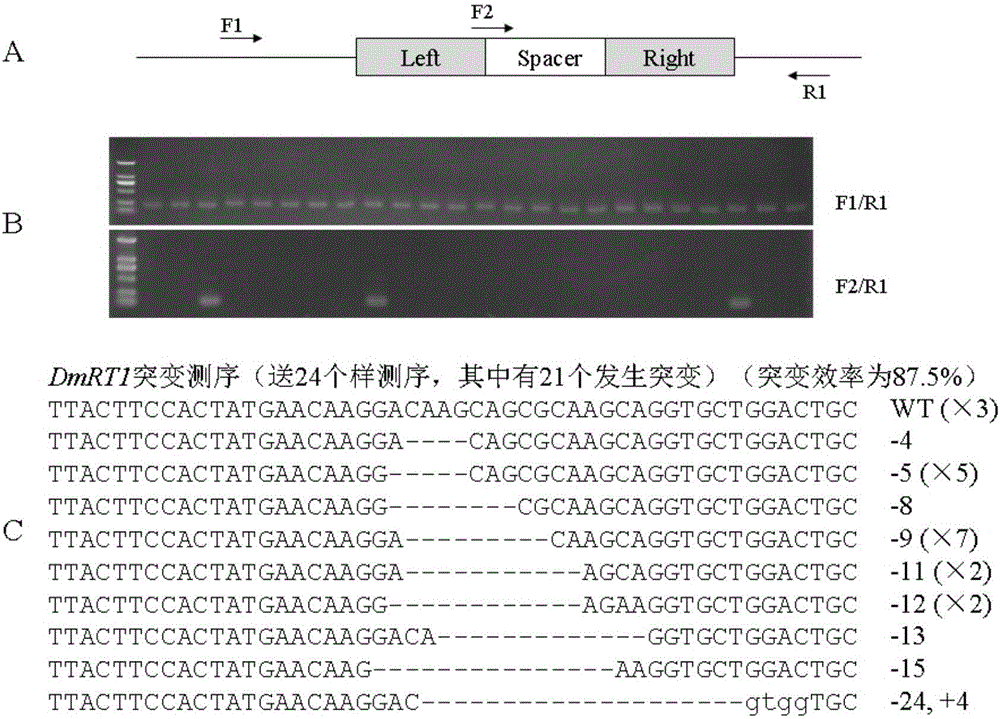
[0046] Example 3, microinjection of rice field eel 1-cell stage embryo
[0047]A layer of 1% agar was poured on the bottom of a 9 cm petri dish, and then 40 fertilized eggs at the 1-cell stage were placed. Under a stereomicroscope (Olympus, Japan), adjust the eel embryo animal pole upward; mix the left and right arms of TALENs mRNA of Dmrt1, so that the final concentration after mixing is 100 ng / μL; use a nitrogen-pressurized quantitative microinjection system (Warner PLI-100A, USA) were injected one by one into the animal poles of fertilized eggs at the 1-cell stage of rice field eel, and the injection volume of each fertilized egg was 2nL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com