Gold-as-core silver-as-shell ''Raman silent zone'' substrate and preparation method and application thereof
A Raman silent zone and Raman substrate technology, applied in Raman scattering, material excitation analysis, etc., to achieve the effect of great clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
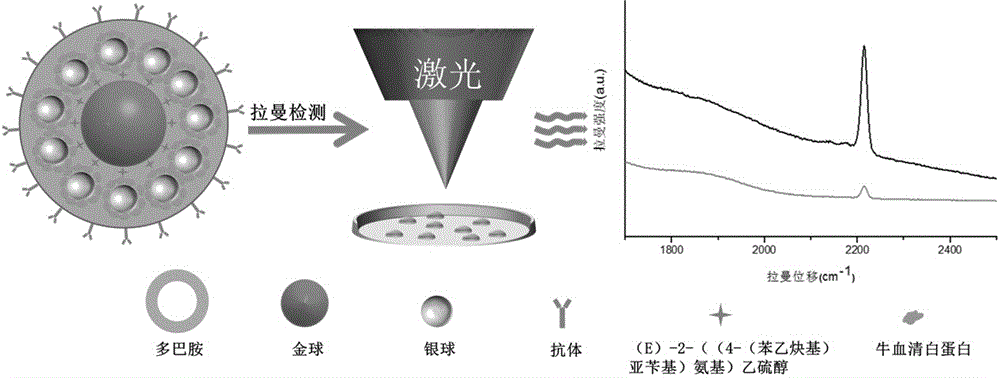
[0024] Example 1: Synthesis of a surface-enhanced Raman scattering cell imaging substrate with gold as the core and silver as the shell modified by human epidermal growth factor receptor antibody
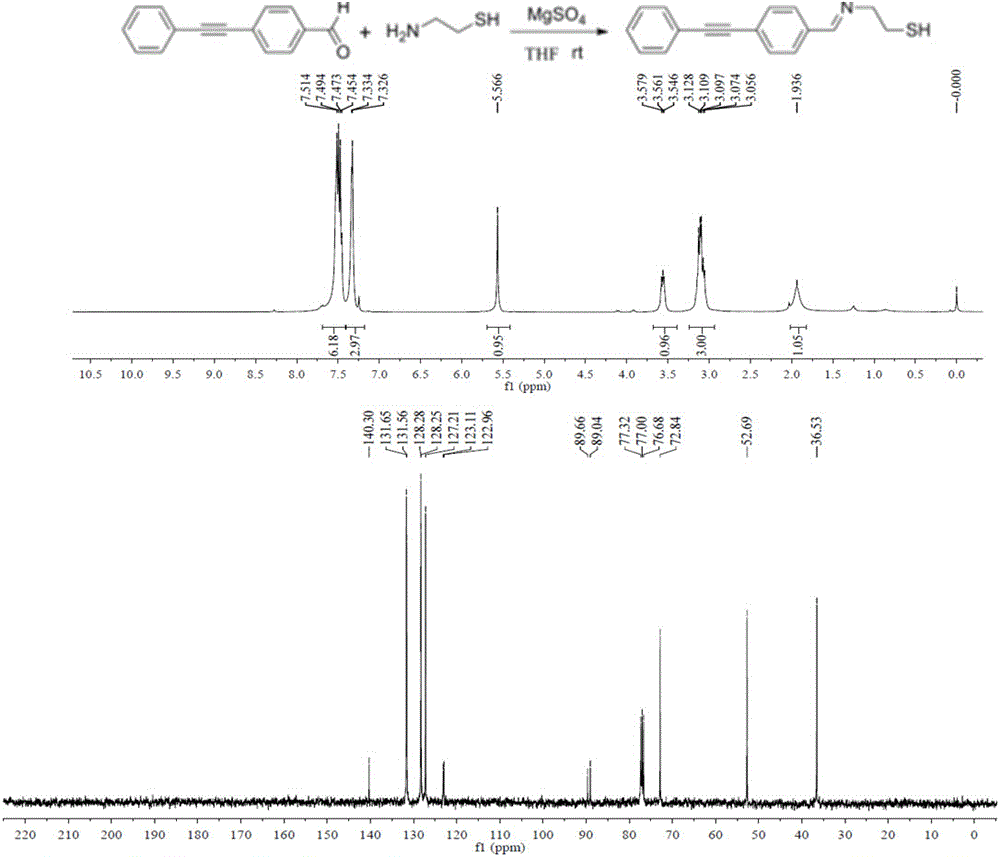
[0025] Pipette 1.212 mL of 10 mg / mL perchlorauric acid solution and 98.788 mL of deionized water into a 250 mL three-necked flask, and add 10 uL of 10 mM (E)-2-((4-(phenylethynyl )benzylidene)amino)ethanethiol, mix it evenly, heat it in an oil bath at 140°C, and control it to reflux about once per second, then add 750 uL of 1% citric acid tris Sodium, maintain reflux for about 30 minutes, cool to room temperature, store it in a clean glass bottle, and store it at 4°C. Take the synthesized gold nanoparticles with (E)-2-((4-(phenylethynyl)benzylidene)amino)ethanethiol molecule, centrifuge it at 8000rpm for 5min, absorb the supernatant, Redissolve in borax solution, add 10 uL of 10 mM (E)-2-((4-(phenylethynyl)benzylidene)amino)ethanethiol to it, mix well and add 5 uL of 0.1 M anti Re...
Embodiment 2
[0026] Example 2: Application of Raman material with gold as core and silver as shell to identify glioma cell U251 and perform Raman imaging on it
[0027] First add about 10 5 After culturing for 12 hours, take 10-30 μL of antibody and gold as the nucleus and silver as the surface-enhanced Raman scattering substrate of the silent zone as the shell, add it to a glass dish, and then put the glass culture dish at room temperature, and Cells were incubated for 1-2 h, then aspirated the medium, washed three times with PBS, reacted with 4% paraformaldehyde solution in a 37°C incubator for 15 minutes, and finally washed three times with PBS, and added 100 uL of PBS to the Store in a refrigerator at 4°C for surface-enhanced Raman scattering imaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com