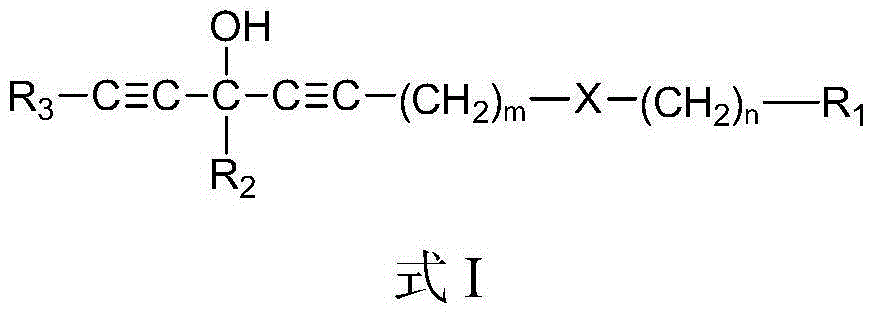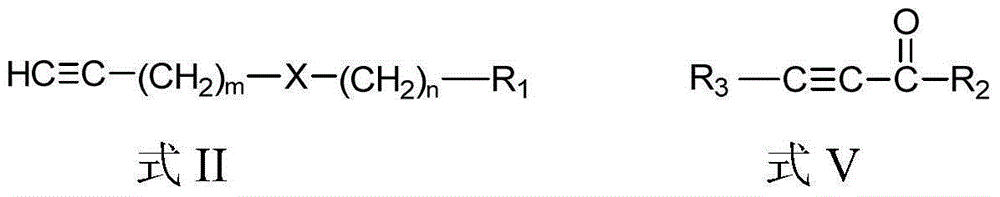Chain polyacetylene compound and preparation method and application thereof
A compound and chain-like technology, applied in the fields of organic chemistry and metalorganic chemistry, to achieve the effects of controllable structure, efficient acquisition, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
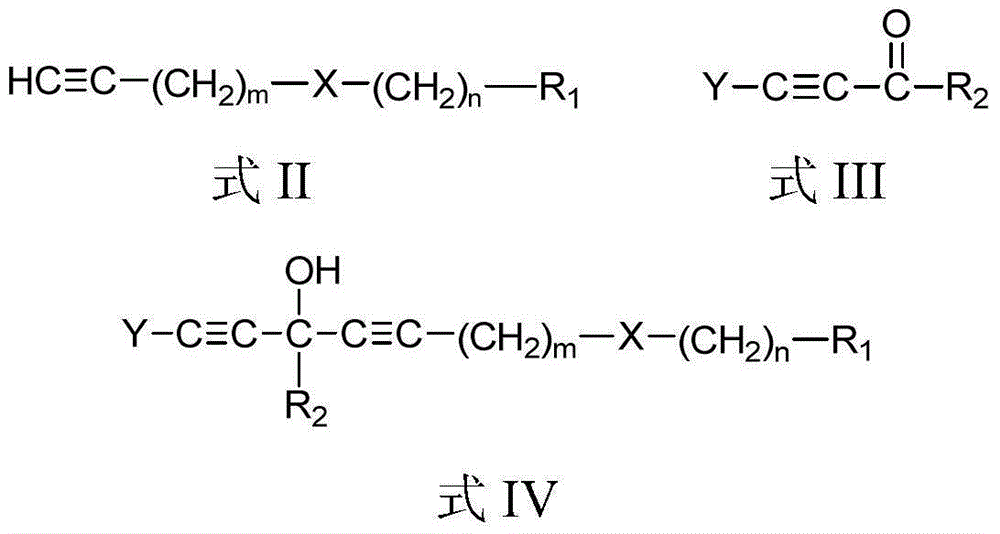
[0070] According to the second aspect of the present invention, the present invention provides the preparation method of above-mentioned chain polyyne compound, when R 3 For hydrogen, the method includes:
[0071] Compound shown in formula II and organometallic reagent RM 1 and / or RM 2 Z carries out the metal exchange reaction in the aprotic solvent, and the obtained reaction mixture is contacted with the compound shown in the formula III to obtain the chain polyyne compound protected by the Y group shown in the formula IV; then the compound shown in the formula IV The chain-like polyyne compound of the Y group protection shown carries out deprotection group treatment, obtains the chain-like polyyne compound shown in formula I:
[0072]
[0073] in,
[0074] Y is a protecting group used to protect an alkynyl group, and Y can be any one of TMS (trimethylsilyl), TES (triethylsilyl) and TIPS (triisopropylsilyl). X, R 1 , m and n are the same as above.
[0075] The organo...
Embodiment 1
[0098] This example is used to prepare the chain polyyne compound provided by the present invention.
[0099]
[0100] Among them, TMS stands for trimethylsilyl. EtMgBr is ethylmagnesium bromide (purchased from Bailingwei Technology Co., Ltd., trade name is 248474), (Trimethylsilylpropynaldehyde, purchased from Bailingwei Technology Co., Ltd., the trade name is 2975-46-4), (n-Bu) 4 NF (tetra-n-butylammonium fluoride, purchased from Bailingwei Technology Co., Ltd., the trade name is A10588).
[0101] (1) Preparation of compound 1
[0102] Under nitrogen atmosphere, under magnetic stirring, 1,6-heptadiyne (5.0mL, 4.02g, 43.63mmol, purchased from Aladdin Reagents (Shanghai) Co., Ltd., trade name H102744-25mL) was dissolved in 100mL THF , cooled to 0°C, added ethylmagnesium bromide solution (1.0M tetrahydrofuran solution, 43.6mL, containing 43.60mmol ethylmagnesium bromide) dropwise, added within 1h, reacted at room temperature for 1h and then cooled to 0 ℃, quickly added ...
Embodiment 2
[0110] This example is used to prepare the chain polyyne compound provided by the present invention.
[0111]
[0112] Wherein, n-BuLi is n-butyllithium (purchased from Bailingwei Technology Co., Ltd., the trade name is 913796).
[0113] (1) Preparation of Compound 3
[0114] Under nitrogen atmosphere, under magnetic stirring, 2,2-dimethyl propargylmalonate (5.00g, 24.0mmol, synthesized according to the document J.Am.Chem.Soc.2013,135,8133.) Dissolve in 200mL tetrahydrofuran, cool to -78°C, add n-butyllithium (2.2M tetrahydrofuran solution, 10.90mL, containing 24.0mmol n-butyllithium) dropwise, add within 1h, react at -78°C for 15min , added trimethylsilylpropynal (3.52mL, 24.0mmol) dropwise, and continued the reaction for 2h. After the reaction, quenched with saturated ammonium chloride solution, extracted with ether, combined the organic phases, dried over anhydrous magnesium sulfate, and filtered , concentrated, and silica gel column chromatography (eluent: n-hexane / et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com