Human parathyrine PTH (1-34) fusion protein and use thereof
A parathyroid hormone and fusion protein technology, applied in the field of medical bioengineering, can solve the problems of difficult and difficult to solve the embedding process, and achieve the effects of long-term biological activity, increase biological activity, and prolong circulation half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the construction of fusion protein and its plasmid
[0039] First, an RNA synthesis company is commissioned to synthesize RNA, and then cDNA is synthesized from RNA using reverse transcriptase. Then different primers were used to amplify the desired PTH (1-34) fragment by polymerase chain reaction (PCR). The Fc fragment of immunoglobulin is obtained by PCR amplification from the cloned IgG1 Fc plasmid. Finally, PCR is used to fuse the PTH(1-34) fragment and the Fc sequence, thereby constructing the DNA sequence of the PTH(1-34)-Fc fusion protein.
[0040] The polymerase chain reaction included: denaturation at 95°C for 30 seconds; annealing at 56°C for 45 seconds; extension at 72°C for 2 minutes, 30 cycles. Using TA cloning kit, clone the PCR products of PTH(1-34) and Fc into pET-32a plasmid respectively, transfect, E.coli BL21(DE3), select white colonies, add LB medium, and culture overnight. Then the plasmid was extracted with Qiangen plasmid extracti...
Embodiment 2
[0054] Example 2: Expression of PTH(1-34)-Fc fusion protein in cells
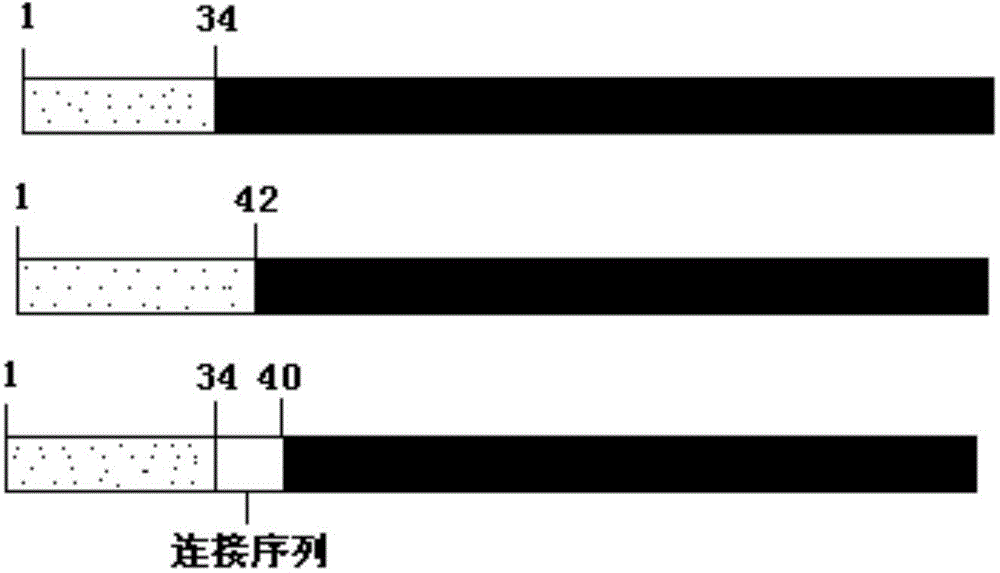
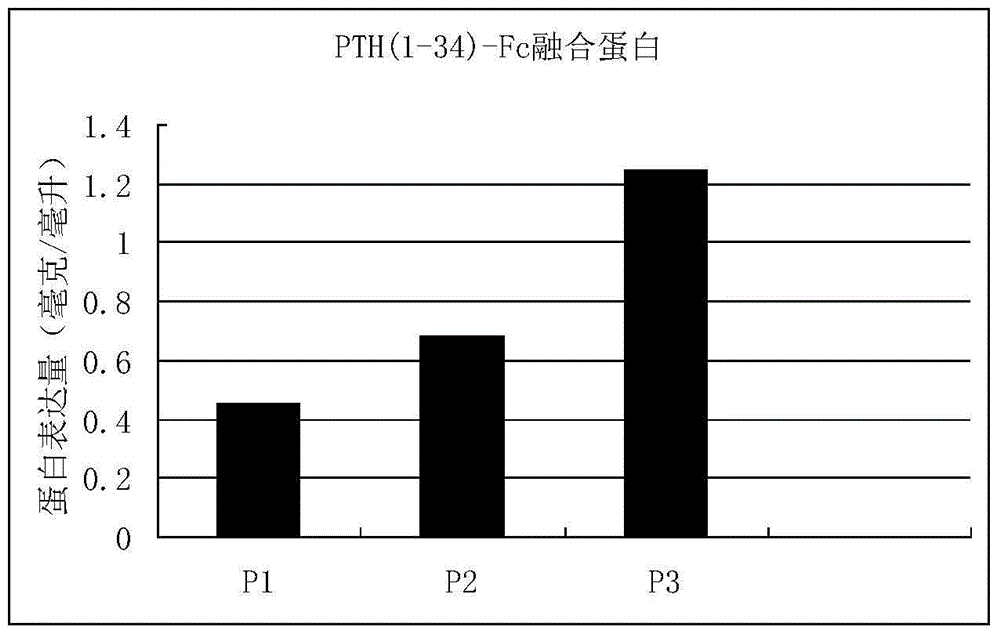
[0055] This example provides an example of expressing PTH(1-34)-Fc fusion protein by taking mammalian cells as an example. Three fusion proteins of P1, P2 and P3 were cloned into pET-32a plasmid (Invitrogen). A human F1t-1 signal peptide sequence was added to the 5' end of the fusion protein. The above three PTH(1-34)-Fc high-purity plasmid DNAs were extracted with a plasmid DNA purification kit (MoBio Company). Then, the plasmid DNA was introduced into CHO cells by using the FUGEN6 transfection kit (ROCHE Company). We compared the expression levels of these four PTH(1-34)-Fc fusion proteins by means of transient transfection. CHO cells were cultured in cell culture dishes with DMEM complete medium containing 10% fetal bovine serum. When the cells grew to a certain point, the complex of plasmid DNA and FUGEN6 reagent (Roche) was added to the cell culture medium, and the supernatant was collected after c...
Embodiment 3
[0056] Example 3: Establishment of high-efficiency expression cell lines
[0057] After confirming that the P3 fusion protein is the best molecular combination, stable and high-efficiency expression cell lines of CHO were established by means of stable transfection and gene amplification. The gene containing the P3 fusion protein was cloned into a plasmid containing the DHFR gene. High-purity plasmid DNA was extracted with a DNA purification kit from MoBio, and then transfected into CHO cells by electrodrilling. The transfected cells were cloned in selective culture to obtain resistant clones. Gene amplification was carried out under pressure with methotrexate (MTX) to obtain highly expressed cell lines. Finally, the cells are expanded to produce the fusion protein on a large scale in a bioreactor. The concentration of fusion protein was determined quantitatively by ELISA. Using this method, the P3 fusion protein was successfully produced on a large scale. The output can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com