Tacrolimus sustained-release tablets and preparation method thereof
A technology for tacrolimus and sustained-release tablets, applied in the field of water-insoluble drug tacrolimus sustained-release tablets and its preparation, can solve problems such as complex processes, achieve simple prescription processes, low production costs, and maintain blood drug concentrations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
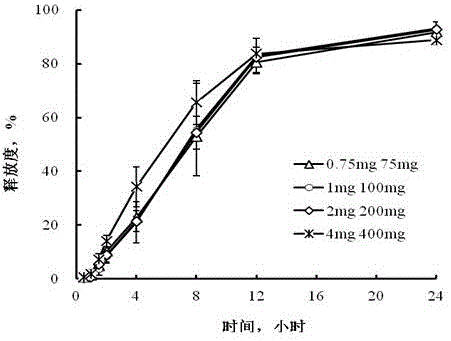
Examples
Embodiment 1
[0049] Embodiment 1: Tacrolimus solid dispersion
[0050] Prescription 1:
[0051] Tacrolimus 10mg
[0052] Glyceryl Behenate 150mg
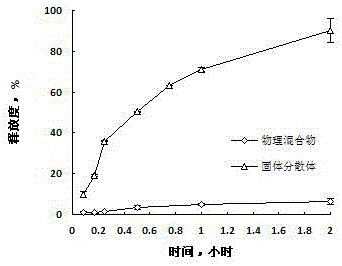
[0053] Weigh glyceryl behenate, heat and stir in a water bath at 80°C to melt, then lower the temperature to 70°C, add tacrolimus, continue stirring for 3 hours, slowly cool to room temperature, crush, pass through a 60-mesh sieve, and place at 40°C Put it down for 24h. 4h dissolution rate was 95%. After being placed at 40°C for 10 days, the dissolution rate in 4 hours was 80%. It shows that the physical stability of the prescription is not good, and the dissolution rate obviously decreases after being placed.
Embodiment 2
[0054] Embodiment 2: tacrolimus solid dispersion
[0055] Prescription 2:
[0056] Tacrolimus 10mg
[0057] Glyceryl Behenate 75mg
[0058] Macrogol Glyceryl Stearate 75mg
[0059] Weigh glyceryl behenate and polyethylene glycol stearate, heat and stir in a water bath at 80°C to melt, then lower the temperature to 70°C, add tacrolimus, continue stirring for 3 hours, slowly cool to room temperature, and pulverize , passed through a 60-mesh sieve, and placed at 40°C for 24 hours. The dissolution rate in 0.5h is 98%; after 10 days at 40°C, the dissolution rate in 0.5h is 20%. It shows that the physical stability of the prescription is not good, and the dissolution rate obviously decreases after being placed.
Embodiment 3
[0060] Embodiment 3: tacrolimus solid dispersion
[0061] Prescription 3:
[0062] Tacrolimus 10mg
[0063] Glyceryl Behenate 125mg
[0064] Stearic acid 25mg
[0065] Weigh glyceryl behenate and stearic acid, heat, stir and melt in a water bath at 80°C, lower the temperature to 70°C, add tacrolimus, continue stirring for 3 hours, slowly cool to room temperature, pulverize, pass through a 60-mesh sieve, Place it at 40°C for 24h. 4h dissolution rate was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com