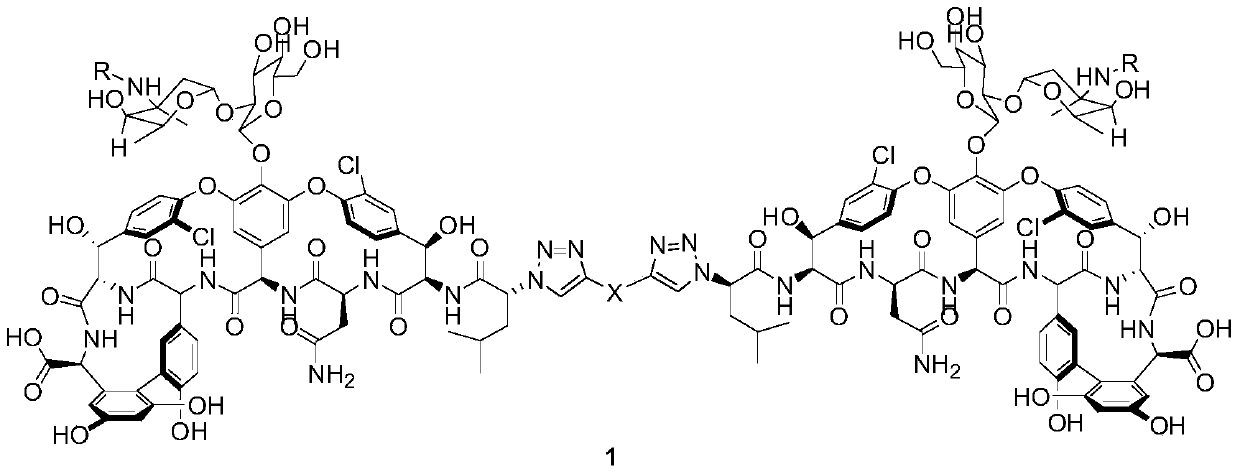
Norvancomycin dimer derivative and its preparation method and medicinal use
A technology of norvancomycin and dimer is applied in the field of medicine to achieve the effect of high antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of norvancomycin dimer derivative 1a
[0026]
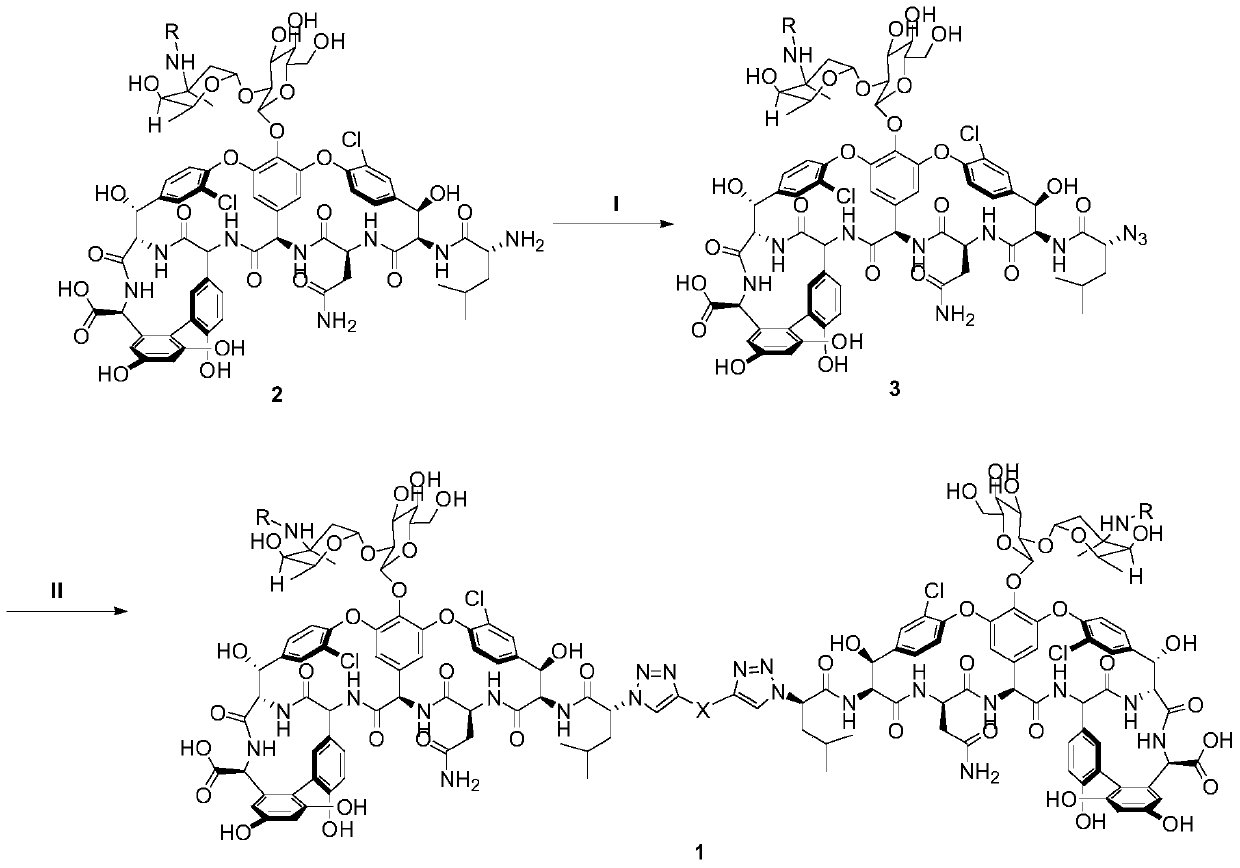
[0027] Lead compound 2a (100mg, 0.062mmol) was added to a 25mL three-neck flask, dissolved in 5mL DMF, and after the temperature was lowered to 0°C, DIEA (16.03mg, 0.124mmol) and 0.1% equivalent of CuSO were added 4 .5H 2 O as a catalyst, under nitrogen protection, TfN was added dropwise at 0 °C 3 The DCM solution was added to the reaction system, and reacted at 0°C for 8h. TLC monitors that the basic reaction of the raw materials is complete. Slowly add DCM dropwise to the system, gradually insoluble matter is produced, continue to drop DCM until no insoluble matter is produced, centrifuge, discard the supernatant, and obtain lavender powdery solid 3a (92 mg , yield 90.7%);
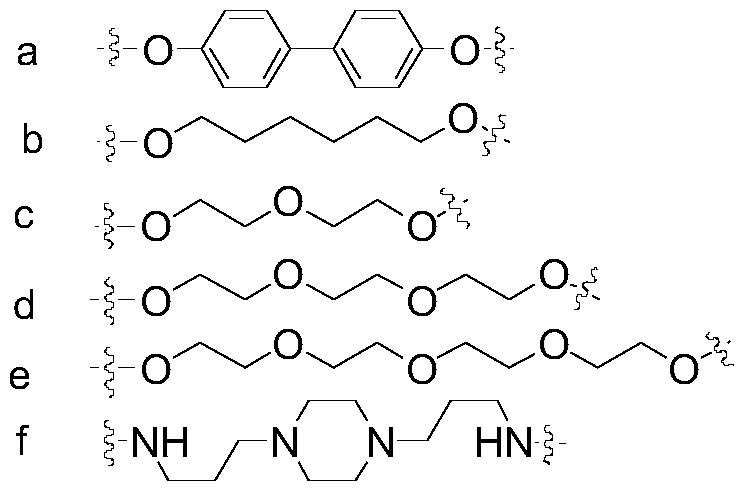
[0028] Add compound 3a (100mg, 0.061mmol) into a 10mL three-neck flask, dissolve it with 5mL DMF, add DIEA (31.6mg, 0.244mmol), after compound 3a is completely dissolved, N 2 Add bridge chain I (8mg, 0.0305mmol) and 0.1% CuI under pr...
Embodiment 2
[0039] The test of embodiment 2 in vitro antibacterial activity
[0040] Adopt the final product (1) a-c of embodiment 1 to carry out the test of in vitro bacteriostatic activity; Concrete method carries out antimicrobial drug minimum inhibitory concentration determination (Minimal Inhibitory concentration MIC) to carry out;
[0041] Take 1ml of antibacterial drugs of different types and different concentrations and pour them into 9cm sterile empty plates, then immediately pour 19ml of sterile M-H agar cooled to about 55°C on the plates, and mix well with the liquid to make the culture medium antibacterial drugs The final concentrations were 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.06 μg / ml; at the same time, M-H plates without antibacterial drugs were prepared as controls; bacterial inoculations were incubated for 18 hours The bacteria were added to sterile normal saline with an inoculation needle to prepare a bacterial solution with a concentration of 0.5 McFarland ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com