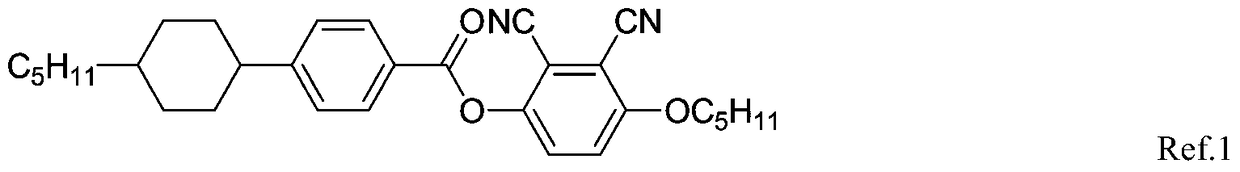
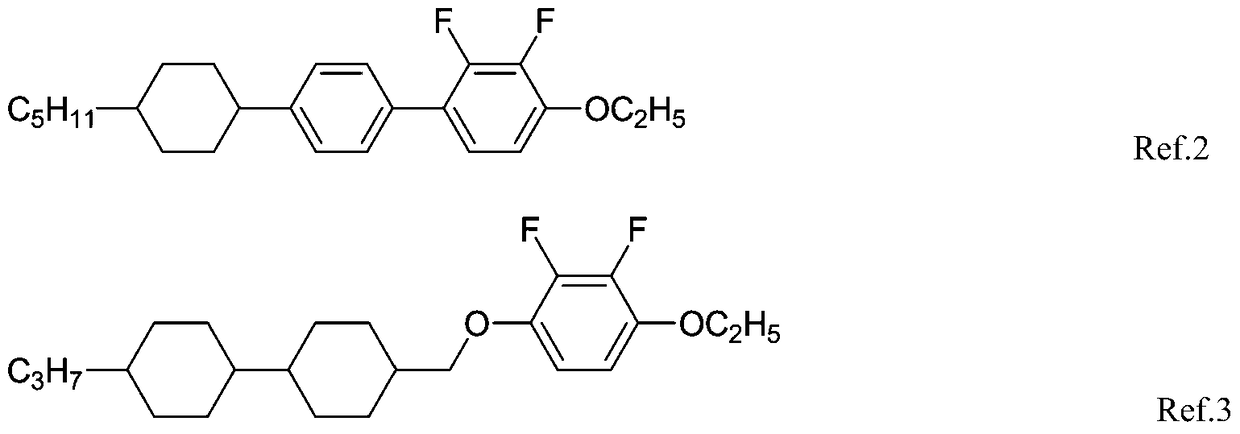
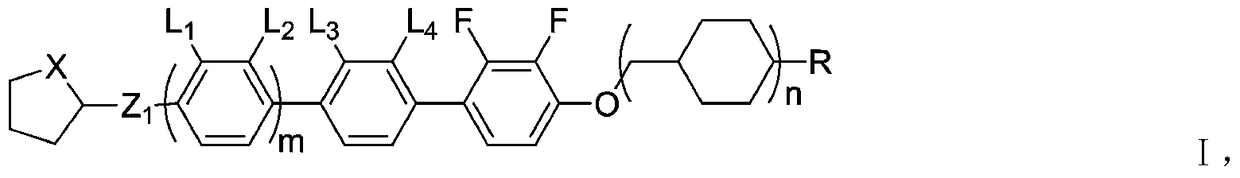
Liquid crystal compound with negative dielectric anisotropy and its synthesis method and application
An anisotropy and compound technology, applied in the field of liquid crystal compounds with negative dielectric anisotropy and their synthesis, can solve the problems of poor photostability, small negative dielectric anisotropy value, and high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Compound Ⅰ-A-1-3-2 synthetic route is as follows:
[0115]
[0116] Among them, compound A, tetrahydrofurfuryl alcohol, and 4-iodo-2,3-difluorophenetole were all from Jiangsu Hecheng New Materials Co., Ltd.
[0117] 1) Synthesis of Compound B
[0118] Add 13g of compound A, 10.2g of tetrahydrofurfuryl alcohol, 31.4g of triphenylphosphine, and 250ml of dichloromethane into a 500ml three-necked flask. Under nitrogen protection, cool down to 0°C, and dropwise add 20.9g of diethyl azodicarboxylate ( DEAD) and 500ml of dichloromethane. After the dropwise addition, the mixture was naturally warmed to room temperature and continued to stir for 12 hours. Post-treatment, column chromatography purified to obtain white solid compound B: 17.9g, yield: 84%, GC> 97%.
[0119]2) Synthesis of Compound C
[0120] Add 17.9g of compound B and 200ml of anhydrous THF into a 500ml three-necked flask, under the protection of nitrogen, cool down to -78°C, add 35ml of n-BuLi n-hexane solu...
Embodiment 2
[0137] Compound Ⅰ-A-2-3-3 synthetic route is as follows:
[0138]
[0139] 1) Synthesis of Compound E
[0140] Add 13g of compound A, 15.6g of propylcyclohexylmethanol, 31.4g of triphenylphosphine, and 250ml of dichloromethane into a 500ml three-necked flask. The mixed solution composed of ester (DEAD) and 500ml of dichloromethane, after the dropwise addition, was naturally warmed to room temperature, continued to stir for 12h, post-processing, and purified by column chromatography to obtain white solid compound E: 22g, yield: 82%, GC >97%.
[0141] 2) Synthesis of Compound F
[0142] Add 22g of compound E and 200ml of anhydrous THF to a 500ml three-necked flask, under the protection of nitrogen, cool down to -78°C, add 35ml of n-BuLi n-hexane solution (2.4mol / L) dropwise, after the dropwise addition, keep at -78°C Stirred for 1h, then added dropwise by 21g I 2 and 100ml of anhydrous TFT, after the dropwise addition, keep stirring at -78°C for 1h, then naturally warm up...
Embodiment 3
[0153] Compound I-A-1-3-2, Compound I-A-2-3-3, Compound I-A-3-3-2 were mixed with the host liquid crystal (host) according to the mass ratio of 10:90, The liquid crystal parameters of Compound I-A-1-3-2, Compound I-A-2-3-3 and Compound I-A-3-3-2 were tested by extrapolation method as shown in Table 3 below:
[0154] table 3
[0155]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com