Chinese medicinal composition for treatment of liver cancer and application thereof
A technology for the treatment of liver cancer, composition, applied in the field of traditional Chinese medicine composition for the treatment of liver cancer, can solve the problems of damage, side effects and toxicity, and achieve extensive social benefits, good effect and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 Chinese medicine extract
[0071] Preparation:
[0072] 1. Weigh the crude drug according to the formula in Table 1;
[0073] 2. Extraction of raw materials: This prescription adopts different extraction processes according to the characteristics of the active ingredients of different drugs in the prescription. Hedyotis diffusa and Panax notoginseng are herbal traditional Chinese medicines, and extraction together is beneficial to the dissolution of active ingredients of Hedyotis diffusa. Hedyotis diffusa is a fat-soluble component. Through the emulsification of notoginseng saponins, the fat-soluble components in Panax notoginseng and Hedyotis diffusa can be extracted greatly without affecting the extraction of water-soluble components. The process is simple The cost is low and the effect is good. Ganoderma lucidum mycelium and Ganoderma lucidum fruiting body are both medicinal fungi, and their active ingredients are polysaccharides and...
Embodiment 2
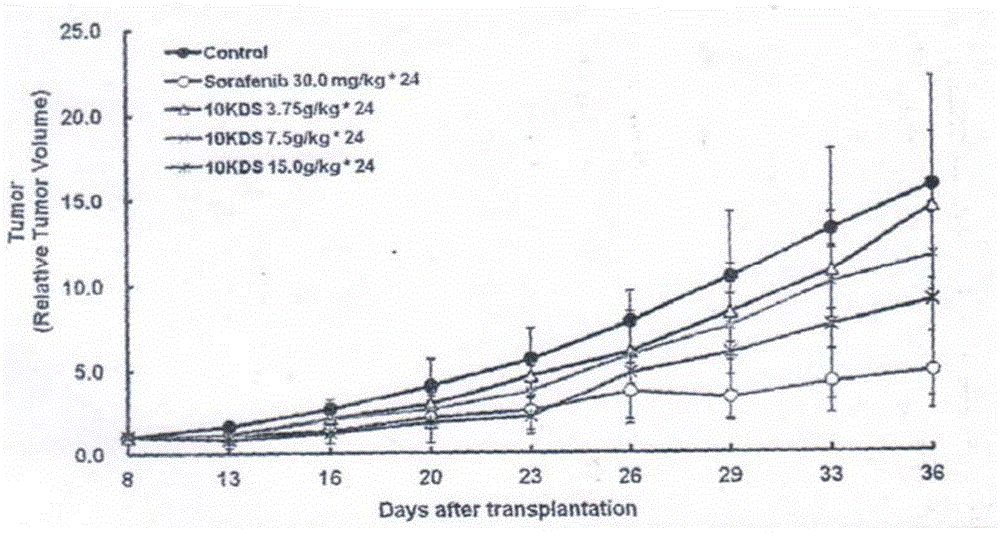
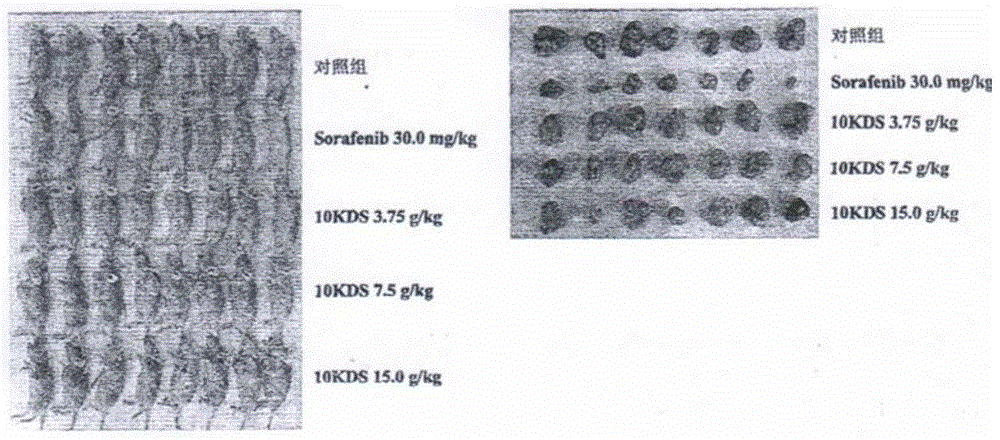
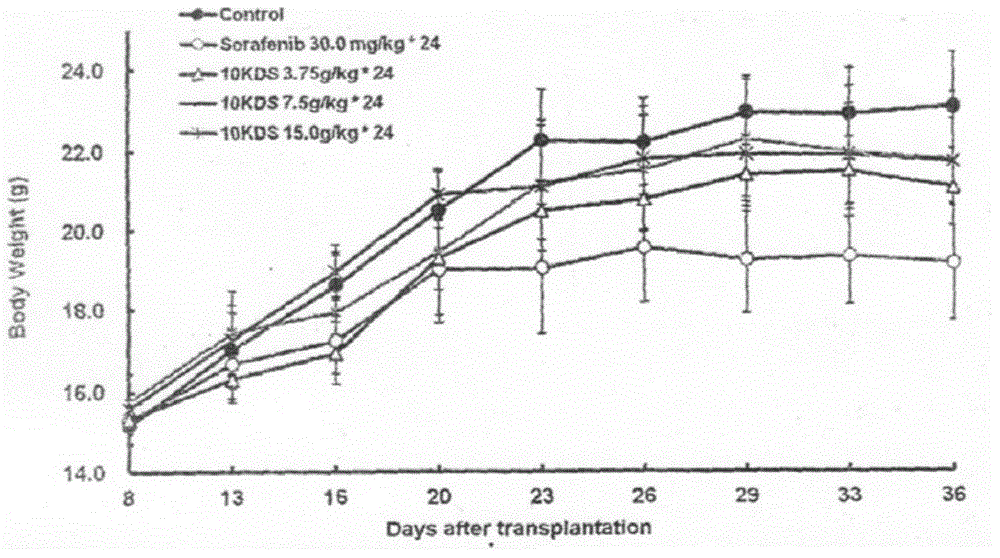
[0081] Example 2 Pharmacodynamics Study: Inhibitory Effect on Liver Cancer HepG2 Nude Mice Transplanted Tumors
[0082] 1. Purpose of the experiment
[0083] The inhibitory effect of the drug prepared according to the method described in Example 1 on human liver cancer HepG2 nude mice xenografts was investigated.
[0084] 2. Experimental animals
[0085] Balb / c / nu nude mice (male, body weight 18-20g; female, body weight 15-18g),
[0086] Provided by Beijing Huafukang Biotechnology Co., Ltd., certificate number: SCXK (Beijing) 2014-0004.
[0087] 3. Experimental Drugs and Reagents
[0088] l. Drugs: prepared by the method described in Example 1, recorded as: 10KDS; Sorafenib: positive control drug, provided by the Institute of Materia Medica, Chinese Academy of Medical Sciences, with a purity of 98.0%.
[0089] 2. Drug preparation method:
[0090] 10KDS: Prepare 0.1875g / m1, 0.375g / m1 and 0.75g / m1 liquid medicine with sterile distilled water, the dosage is 3.75g / kg, 7.5g / kg...
Embodiment 3
[0122] The toxicity study of embodiment 3 prescription
[0123] The medicine prepared according to the method described in Example 1 was subjected to acute toxicity test. The method adopted is the maximum dose test, the test selects the administration route to be recommended for clinical trials, and the animal tolerates the maximum concentration and the maximum volume of the dose once or 2-3 times a day. Take oral administration of mice as an example: the number of animals shall not be less than 20, half male and half male, the volume shall not exceed 0.4ml / g body weight at one time, and the interval between multiple administrations shall not be less than 4h. Observe for 14 days and record the toxic reaction and body weight change. If there is still no toxic reaction or normal weight gain, it can be considered that the drug has no toxic reaction, or the LD50 is greater than the maximum dose.
[0124] In this experiment, the maximum dose of 10KDS13 administered orally to mice ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com