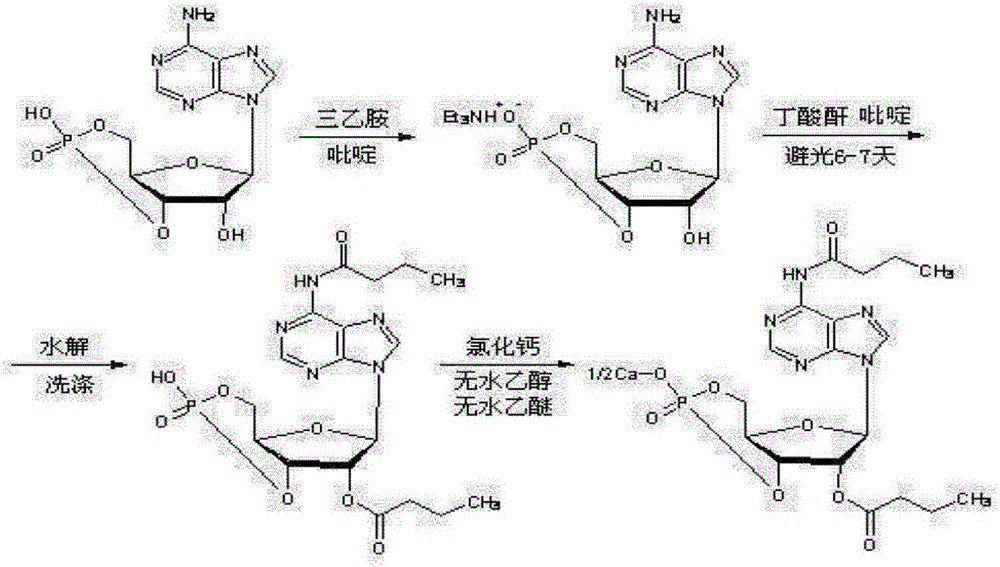
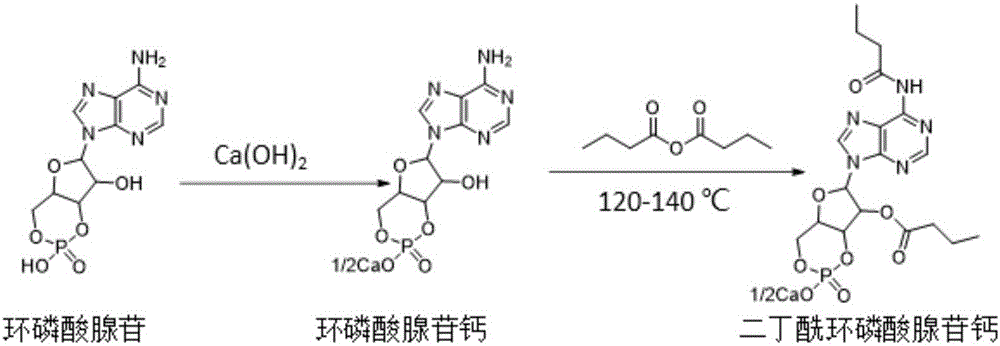
Calcium dibutyryladenosine cyclophosphate preparation method
A technology of calcium dibutyryl cyclic adenosine phosphate and calcium cyclic adenosine phosphate, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of cumbersome process, poor product quality, and consumption cost, and achieve High reaction selectivity, short reaction time, and cost-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Accurately weigh cyclic adenosine monophosphate and calcium hydroxide in a beaker, and add a certain amount of water to the beaker, wherein the molar ratio of cyclic adenosine monophosphate and calcium hydroxide is 1:1.2, the mass of cyclic adenosine monophosphate and water Ratio 1:6, vigorously stirred for about 30 minutes, filtered the reaction solution, and removed the filter residue.
Embodiment 2
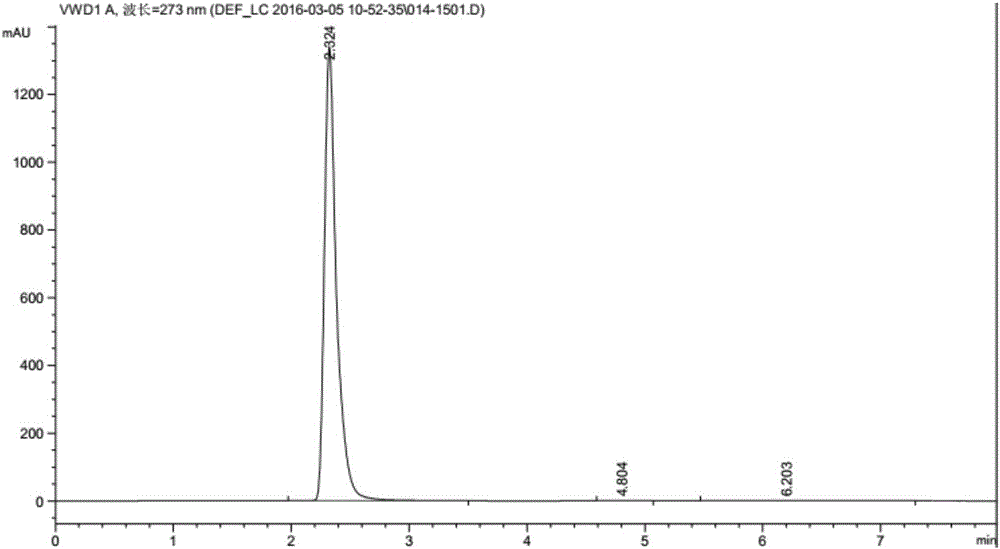
[0038] To the above filtrate, add ethanol dropwise to crystallize and stir vigorously, the volume ratio of ethanol to filtrate is 3:1, filter the precipitated cyclic adenosine monophosphate calcium salt, and vacuum dry at 60-80°C to obtain the product cyclic adenosine monophosphate Calcium salt, the conversion rate is 100%, and the selectivity is >99%. The liquid phase diagram of calcium cyclic adenosine monophosphate is shown in image 3 .
Embodiment 3
[0040] The acylation reaction is carried out in a three-necked flask. One of the three-necked flasks is connected to a condenser, the other is protected by nitrogen gas, and the other is fed with materials. The reaction is heated in an oil bath and stirred by a magnetic stirrer.
[0041] The reaction device is as shown above, weigh a certain amount of cyclic adenosine monophosphate calcium in the flask, and add a certain volume of butyric anhydride to it, wherein the mass volume ratio of cyclic adenosine monophosphate calcium and butyric anhydride is 1g:10ml, react respectively React at 120° C., 130° C., and 140° C. for 3 hours. After the reaction, samples were taken for detection. The conversion and selectivity are shown in Table 1.
[0042] Selectivity and conversion rate of table 1 embodiment 3
[0043] temperature reflex Conversion rates selectivity 120℃ 63.2% 87.4% 130℃ 89.7% 92.4% 140℃ 97.6% 94.6%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com