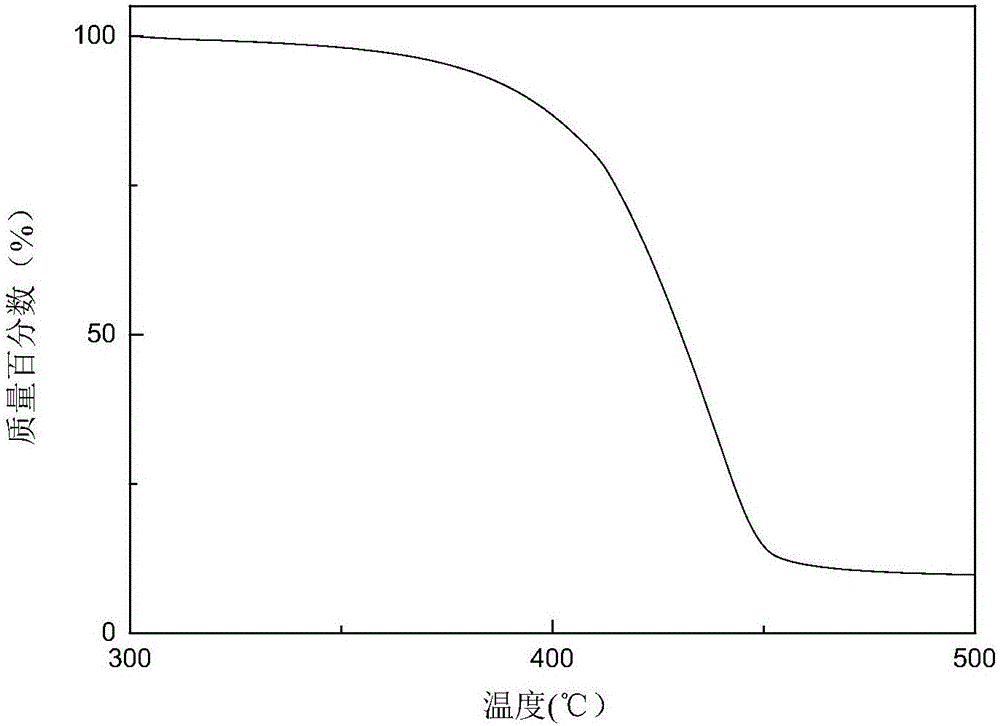
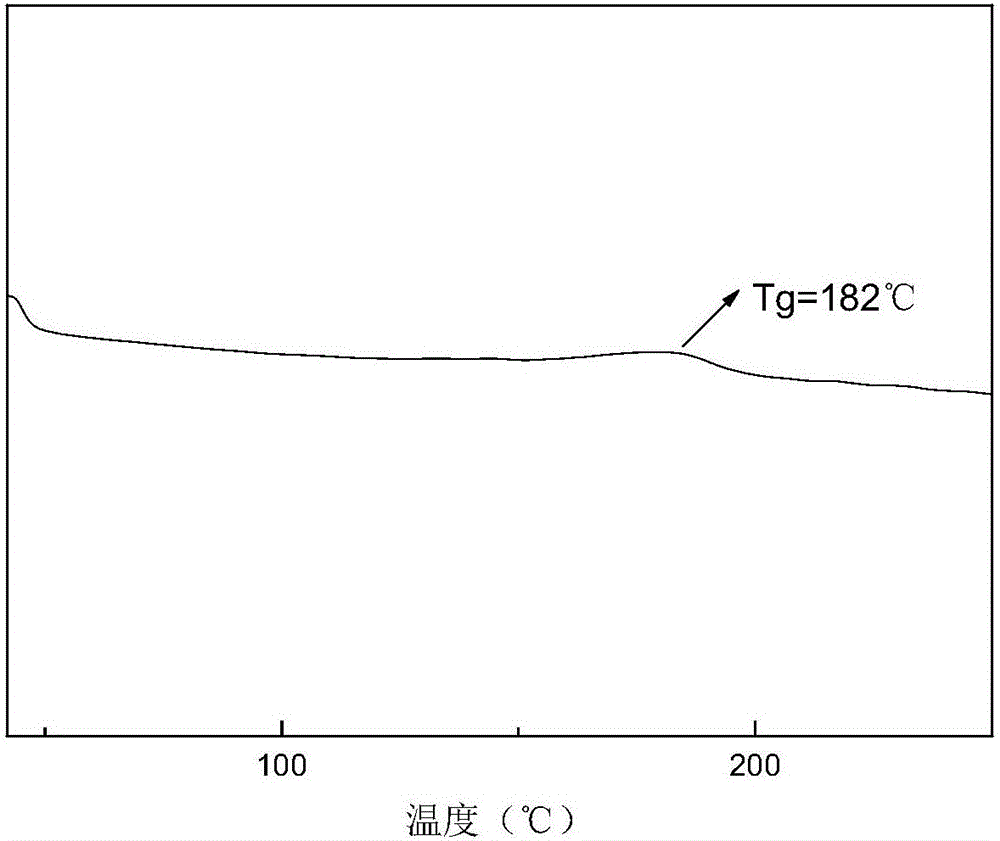
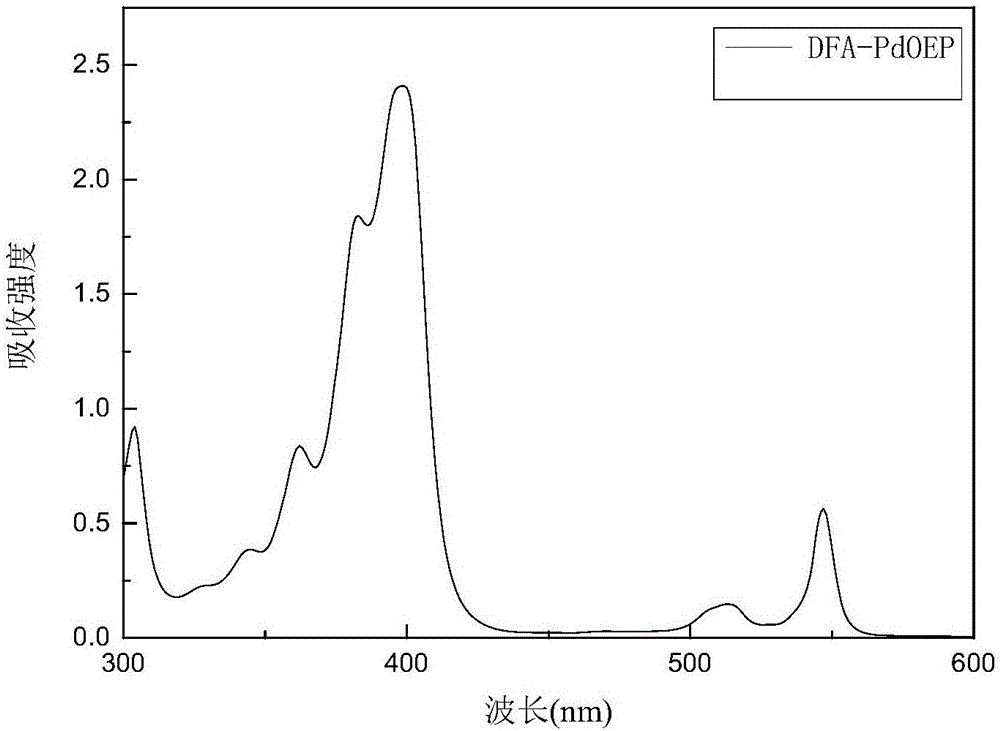
Annihilation agent applied to triplet-triplet annihilation up-conversion system, and preparation and application methods thereof
A technology of triplet annihilation and triplet state, which is applied in the preparation of organic compounds, carbon-based compounds, chemical instruments and methods, etc., can solve the problems of low fluorescence quantum yield, few types of annihilation agents, poor stability, etc., and achieve Efficient and stable preparation, pollution-free preparation process, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 9,10-difluorenylanthracene (DFA).
[0031] Respectively mix 5mmol of phenylboronic acid, 5mmol of pro-bromobenzaldehyde and 5mmol of K 2 CO 3 Add to 12ml DMF / H2O solution, stir for 2min until fully dissolved, add 5mol% Pd(OAc)2, fill with nitrogen protection, stir at 25℃, reaction stop for 5h, add 50ml acetic acid to the solution, wash ethyl acetate with water The ester was extracted 3 times, dried with anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by column chromatography (silica gel, petroleum ether: ethyl acetate 50:1) to obtain a colorless liquid product.
[0032] Add 10mmol 2-aldehyde biphenyl and 5mmol anthracene into a 250ml single-neck flask filled with 100ml dichloroethane, add 10mmol acetic anhydride while stirring, add 0.5mmol trifluoromethanesulfonic acid dropwise, stir at 25℃, TLC (expanded Petroleum ether: ethyl acetate=30:1) It is detected that the reaction is over. It was ...
Embodiment 2
[0041] Synthesis of 9,10-difluorenylanthracene (DFA).
[0042] Respectively mix 5mmol of phenylboronic acid, 5mmol of pro-bromobenzaldehyde and 5mmol of K 2 CO 3 Add to 12ml DMF / H2O solution, stir for 2min to fully dissolve, add 5mol% Pd(OAc)2, fill with nitrogen protection, stir at 25℃, and stop the reaction for 8h, add 50ml acetic acid to the solution, wash ethyl acetate with water The ester was extracted 3 times, dried with anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by column chromatography (silica gel, petroleum ether: ethyl acetate 50:1) to obtain a colorless liquid product.
[0043] Add 10mmol 2-aldehyde biphenyl and 5mmol anthracene into a 250ml single-neck flask filled with 100ml dichloroethane, add 10mmol acetic anhydride while stirring, add 0.5mmol trifluoromethanesulfonic acid dropwise, stir at 25℃, TLC (expanded Petroleum ether: ethyl acetate=30:1) It is detected that the reaction is over. It ...
Embodiment 3
[0045] Synthesis of 9,10-difluorenylanthracene (DFA).
[0046] Respectively mix 5mmol of phenylboronic acid, 5mmol of pro-bromobenzaldehyde and 5mmol of K 2 CO 3 Add to 12ml DMF / H2O solution, stir for 2min to fully dissolve, add 5mol% Pd(OAc)2, fill with nitrogen protection, stir at 25℃, reaction stop for 12h, add 50ml acetic acid to the solution, wash ethyl acetate with water The ester was extracted three times, dried with anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by column chromatography (silica gel, petroleum ether: ethyl acetate 50:1) to obtain a colorless liquid product.
[0047] Add 10mmol 2-aldehyde biphenyl and 5mmol anthracene into a 250ml single-neck flask filled with 100ml dichloroethane, add 10mmol acetic anhydride while stirring, add 0.5mmol trifluoromethanesulfonic acid dropwise, stir at 25℃, TLC (expanded Petroleum ether: ethyl acetate=30:1) It is detected that the reaction is over. It was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com