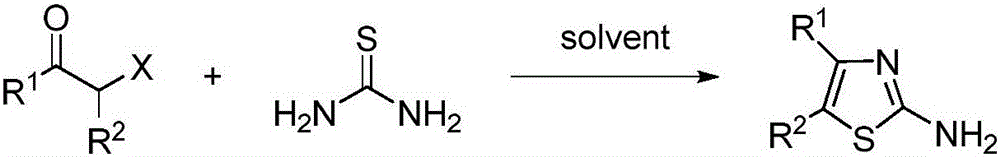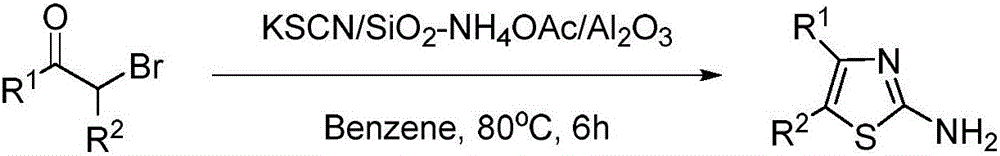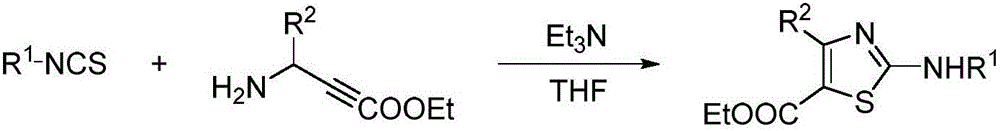Preparation method of 4, 5-disubstituted-2-aminothiazole compound
A technology of aminothiazole and compound, which is applied in the field of compound synthesis, can solve the problems of difficult acquisition of raw materials, low product yield, cumbersome operation, etc., and achieve the effect of novel structure, high reactivity, and simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0052] Example 1-1, 4-methyl-5-(4-bromophenyl)-2-aminothiazole (m1)
[0053]Add 210.1 mg (2.5 mmol) of 50% aqueous cyanamide solution and 480.4 mg (1 mmol) of sodium sulfide nonahydrate into the reaction flask, then add 2.0 mL of n-propanol, 2-methyl-2-nitro-3-( 128.5 mg (0.5 mmol) of 4-bromophenyl) oxirane. After the addition, the reaction was stirred at room temperature for 8 hours, and the reaction was detected by TLC (dichloromethane:methanol=20:1 volume ratio). At this time, the TLC detection reaction result was that 2-methyl-2-nitro-3-(4-bromophenyl)oxirane disappeared, indicating that the reaction had ended.
[0054] After the reaction was finished, concentrate to remove n-propanol, cool to room temperature, add 60mL of water, extract the reaction solution three times with 3×20mL ethyl acetate, wash the organic layer (at the upper layer) three times with 3×30mL saturated brine, and then wash with Anhydrous sodium sulfate (2.0g) was dried for 30 minutes, concentrated by...
Embodiment 1
[0062] Embodiment 1-2, sodium sulfide nonahydrate is changed into sodium hydrosulfide, and the molar weight is constant; all the other are the same as in embodiment 1. 73.7 mg of the product 4-methyl-5-(4-bromophenyl)-2-aminothiazole was obtained as a white solid, with a yield of 55%.
Embodiment 1-3
[0063] Embodiment 1-3, replace n-propanol with methanol, the volume is constant; all the other are the same as embodiment 1. 96.5 mg of the product 4-methyl-5-(4-bromophenyl)-2-aminothiazole was obtained as a white solid, with a yield of 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com