Compound based on indacenodithiophene and application of compound
A compound and mixture technology, applied in the field of organic solar cells, can solve the problems that restrict the development of organic polymer solar cells, the cost of preparation process cannot be reduced, and the absorption in the visible region is weak, so as to improve charge mobility, electron mobility, The effect of superior planar structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] Compounds of formula (I) can be prepared using compounds of formula (II). Therefore, the present invention also provides a preparation method of the compound of formula (I), the method comprising: making R be CHO of the compound of formula (II) reacting in a solvent with a compound selected from the following formula:
[0081]
[0082] Thus the compound of formula (I) is prepared.
[0083] The reaction can be carried out in one or more organic solvents among dichloromethane, chloroform, tetrahydrofuran, chlorobenzene and o-dichlorobenzene. Anhydrous pyridine solution can be added dropwise during the reaction.
[0084] In a specific embodiment, the compound of formula (II) in which R is H is added to a solvent (such as tetrahydrofuran), and after the temperature of the mixture is lowered to below -60°C (such as -78°C), a catalyst (such as n-butyl Lithium) After reacting for 0.5 to 1.5 hours, the mixed solution was warmed up to room temperature, and after stirring fo...
Embodiment 1
[0100] Embodiment 1: Syntheses based on the IDTIDT-IC of andindaprodithiophene
[0101] 1. Synthesis of IDTIDT
[0102]
[0103] In a 150mL round bottom flask, add 2-bromo-4-hexylbenzene (2mol) and tetrahydrofuran (100mL) under the protection of argon, cool the mixed solution to -78 degrees Celsius, and then slowly add n-butyllithium solution dropwise to it (2mol). After the dropwise addition was complete, stir at -78°C for 60 minutes. Then, reactant 1 (0.2 mol) was dissolved in 100 mL of tetrahydrofuran and slowly added dropwise to the mixed solution. After the dropwise addition, the mixed solution was raised to room temperature and continued to stir for 4 hours. After the reaction is complete, extract with dichloromethane, wash with water, dry with anhydrous sodium sulfate, and spin dry the solvent. The obtained crude product is dissolved in 200 mL of acetic acid solution, and after reflux for 30 minutes, 1 mL of concentrated sulfuric acid is added dropwise to the mixtu...
Embodiment 2
[0119] Example 2: Solubility of IDTIDT-IC
[0120] The IDTIDT-IC of Example 1 was placed in several common organic solvents, such as chlorobenzene, dichlorobenzene, chloroform, toluene, trichlorobenzene, methanol, etc. The material was found to have good solubility in chlorinated solvents, but was insoluble in methanol.
[0121] High-quality films can be prepared by spin-coating IDTIDT-IC dichlorobenzene solution on glass slides.
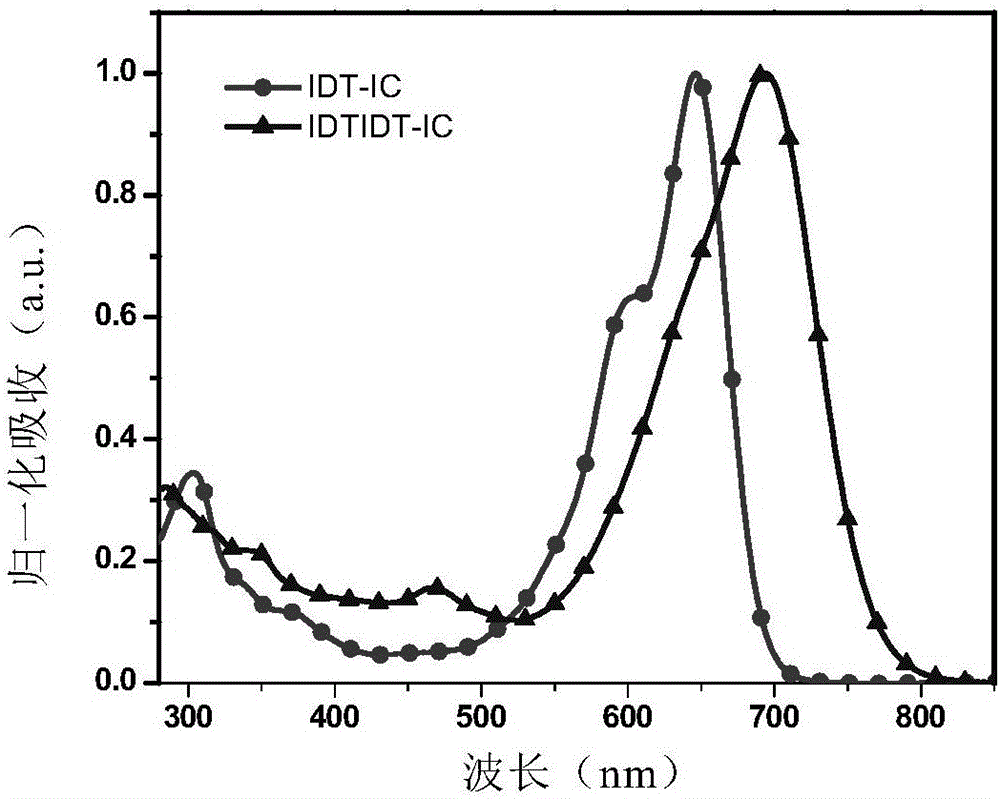
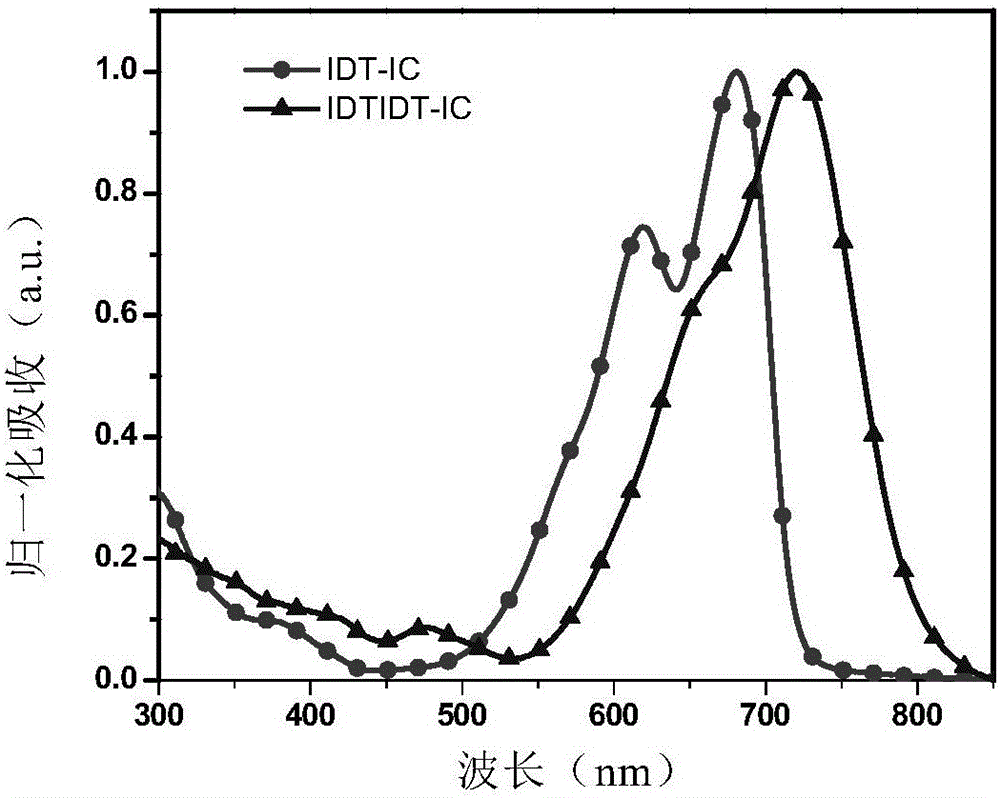
[0122] The absorption spectra of IDTIDT-IC measured under chloroform solution and film are shown in figure 1 and figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com