Novel quality control method applied to immunochromatography test paper strip
A quality control method, immunochromatography technology, applied in the direction of analyzing materials, biological testing, material inspection products, etc., can solve the problems of the difference in the coating of the detection line, the influence of the accuracy of the chromatography speed test cannot be ruled out, and simplify the production Effects of processing steps, improving accuracy and precision of measurement, shortening length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of different wavelength detection molecules and quality control molecule complexes
[0022] 1.1 Preparation of wavelength I detection molecular complex: take 5 mg carboxyl nano fluorescent microsphere I (particle size is 20nm ~ 500nm) according to the conversion of solid content 5mg / ml, add EDC, and its molar content is 1 to 10 times the carboxyl molar content After mixing evenly, shake and react for 30 minutes. After the activation, centrifuge and wash the microspheres with 500 μL of 50 mM PBS solution (pH=7.5). Streptavidin was diluted to 1 mg / mL with 100 mM sodium bicarbonate buffer (pH=8.0), added to the microsphere solution, and placed on a shaker at 220 rpm at 37° C. for 3 h. After the reaction, use 400 μL of 50 mM PBS solution (pH=7.5) to wash the suspended microspheres for three times, and finally add the nanoparticle solution with an amount of 50-500 ug / 100 ul of biotinylated antibody. React for 30-60 minutes, then wash the microspheres ...
Embodiment 2
[0024] Example 2: Preparation of novel immunochromatographic test strips
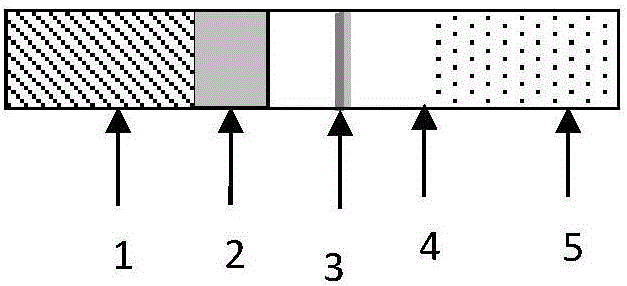
[0025] Such as figure 2 As shown, a novel immunochromatographic test strip includes a bottom plate 1, on which a sample pad 2, a binding pad 3, a nitrocellulose membrane 4 and a water-absorbing pad 5 are pasted in sequence; wherein the sample pad 2, the binding pad 3 , The nitrocellulose membrane 4 and the water-absorbing pad 5 are overlapped by 1 to 2 mm at the joints to ensure that the test sample passes through the binding pad from the sample area to the detection area smoothly. The preparation method of each part is as follows:
[0026] 2.1 Preparation of sample pad 2: Dissolve 0.5g of BSA protein with 100mM, pH7.2-7.4 PBS buffer solution 100mL, then add 0.01-0.05g of surfactant Tween20 to adjust the pH to 6-8; the sample pad is optional Glass fiber or polyester material, a 30cm long sample pad is placed in 2-5ml of the above-mentioned buffer solution, surfactant and protein mixed solution, soaked...
Embodiment 3
[0032] Example 3: Performance Evaluation of Fluorescence Immunochromatography Diagnostic Reagents
[0033] 3.1 Anti-interference experiment of quality control molecules of different physical examination samples
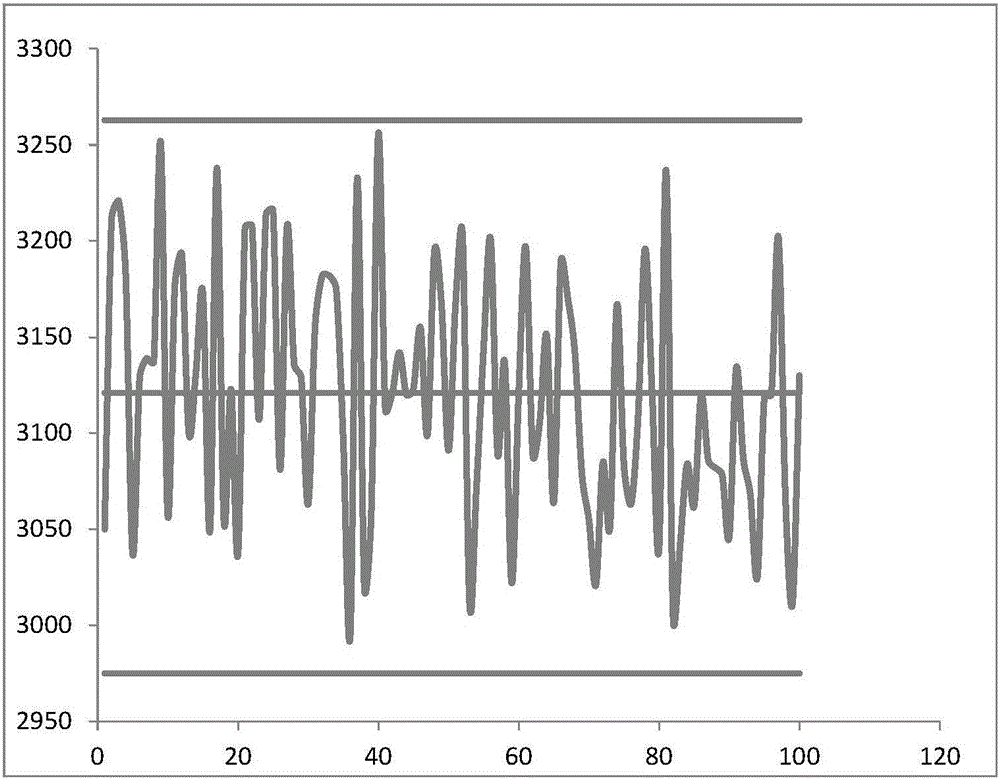
[0034]Randomly select 80 cases of negative serum and 20 cases of positive serum samples, and use the same sample to drop 100ul / root onto the test strip. For 10 minutes, the fluorescence value of the quality control molecular complex was measured with a Getein 1100 multicolor fluorescent immunoassay analyzer (Nanjing Genetic Biotechnology Co., Ltd.). The measured values of 100 samples, the average value of the sample fluorescence, the average + SD and the average - SD are plotted, and the results are shown in image 3 :
[0035] Such as image 3 As shown, unlike the traditional goat secondary antibody and rabbit secondary antibody which are greatly interfered by different samples, the fluorescence values of the new quality control molecule in different serum qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com