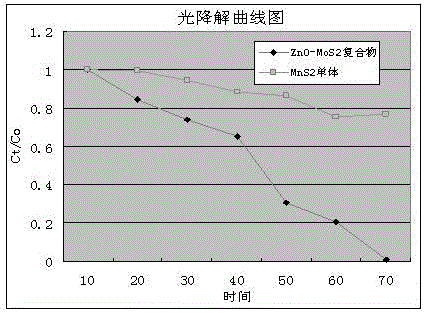
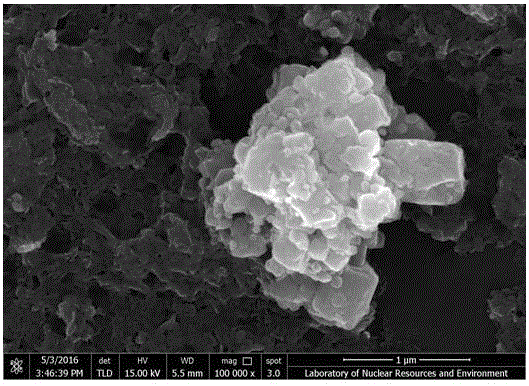
Preparation method of ZnO/MoS2 nanosheet compound photocatalyst
A photocatalyst and nanosheet technology, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of rapid decay of photocatalytic performance and reduction of photocatalytic performance, and achieve significant photocatalytic performance. The effect of performance, simple preparation method and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In beaker A and beaker B, measure 6mL dehydrated ethanol and 7mL deionized water mixing forming agent respectively, then weigh 2mmol zinc nitrate hexahydrate (Zn(NO 3 ) 2 •6H 2 O) and 0.2mmol sodium lauryl sulfate were added to beaker A, placed on a magnetic stirrer and stirred evenly; then weighed 2mmol urea (NH 2 CONH 2 ) into beaker B, and also placed on a magnetic stirrer to stir evenly; then add the solution in beaker B to beaker A drop by drop and keep stirring, then pour the solution in beaker A into 50mL Teflon-lined In a stainless steel autoclave, the autoclave was placed in a 100°C electric blast drying oven for 6 hours, after which the autoclave was naturally cooled to room temperature. Impurities were then removed by centrifugal washing with absolute ethanol and distilled water. Finally, the precipitated product after centrifugation was dried in a vacuum drying oven at 60°C for 12 hours; after that, it was calcined in a box-type resistance furnace at 400...
Embodiment 2
[0027] Take by weighing 100mg sodium molybdate dihydrate and 200mg thioacetamide and add in 100mL beaker A; Get the zinc oxide nanosheet 6mg prepared in example 1, ZnO / MoS 2 Add ZnO to A at a doping ratio of 10% to form B; add 50% deionized water to B and stir on a magnetic stirrer to form C; pour the evenly stirred solution C into the reactor; put the reactor into the electric heating Raise the temperature in a blast drying oven, set the temperature to 220°C, and heat up for 24 hours; after the reaction is over, wait for the reaction kettle to reach room temperature naturally, and then centrifuge 2-3 times with deionized water; centrifuge the precipitate in step 6 Put it in the refrigerator for freezing to freeze the deionized water; put the frozen solid in a freeze dryer for freeze-drying to obtain 10% ZnO / MoS 2 nanosheet composites.
Embodiment 3
[0029] Weigh 100mg of sodium molybdate dihydrate and 200mg of thioacetamide into 100mL beaker A; take 3mg of zinc oxide nanosheets prepared in Example 1, first grind ZnO, and then ZnO and MoS 2 Weigh ZnO at 5% and add it to A to form B; add 50ml deionized water to B and stir on a magnetic stirrer to form C; pour the evenly stirred solution C into the reaction kettle; put the reaction kettle into the electric heating drum Heat up in an air drying oven, set the temperature to 220°C, and heat up for 24 hours; after the reaction is over, wait for the reaction kettle to reach room temperature naturally, and then centrifuge 2-3 times with deionized water; put the centrifuged sediment into the refrigerator Freezing is performed to freeze deionized water; the frozen solid is placed in a freeze dryer for freeze drying to obtain 5% ZnO / MoS 2 Nanosheets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com