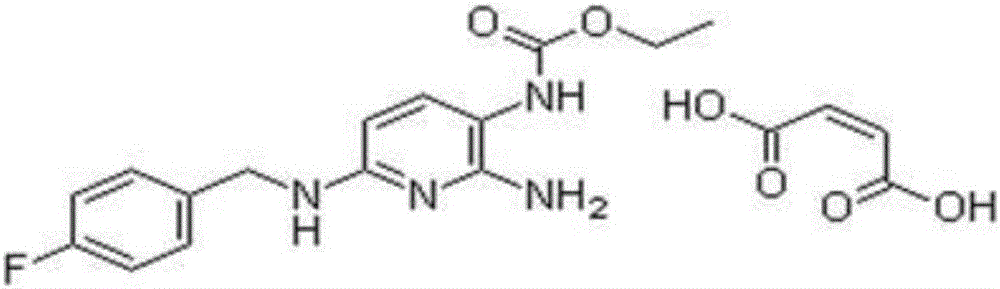
Rivastigmine hydrogen tartrate preparation method and application thereof
A technology of flupirtine maleate and pyridine is applied in the preparation field of flupirtine maleate, can solve the problems of high synthesis cost, difficult to control synthesis conditions, etc., achieves low production cost, strong controllability of preparation process, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of flupirtine maleate includes the following steps:
[0025] A. Synthesis of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine
[0026] Add 10 kg of isopropanol and 3.2 kg of p-fluorobenzylamine into the reaction kettle, stir, add 4 kg of 2-amino-3-nitro-6-chloropyridine and 12.8 kg of triethylamine, and reflux at 85-110°C for reaction 5- After 6h, cooling to 60℃, sampling to monitor the reaction is complete, adding 1kg sodium hydroxide solution, concentrating under reduced pressure until no solvent flows out, centrifuging to obtain a solid, and drying to obtain 2-amino-3-nitro-6-(p-fluorobenzyl) Amino) pyridine;
[0027] B. Synthesis of 2-amino-3-carbamic acid ethyl ester-6-(p-fluorobenzylamino)pyridine
[0028] Add 24.3kg of isopropanol, 5.8kg of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine and 0.5kg of activated carbon to impregnate palladium salt in the autoclave, seal the autoclave, and then pass in argon and hydrogen. , Where the hydrogen pressure ...
Embodiment 2
[0032] A preparation method of flupirtine maleate includes the following steps:
[0033] A. Synthesis of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine
[0034] Add 10kg of isopropanol and 6.8kg of p-fluorobenzylamine into the reaction kettle, stir, add 4kg of 2-amino-3-nitro-6-chloropyridine and 10kg of triethylamine, reflux for 5-6h at 85-110℃ After cooling to 60℃, sampling and monitoring the reaction is complete, add 1kg sodium hydroxide solution, concentrate under reduced pressure until no solvent flows out, centrifuge to obtain a solid, and dry to obtain 2-amino-3-nitro-6-(p-fluorobenzylamine) 基)pyridine;
[0035] B. Synthesis of 2-amino-3-carbamic acid ethyl ester-6-(p-fluorobenzylamino)pyridine
[0036] Add 24.3kg of isopropanol, 5.8kg of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine and 0.5kg of activated carbon to impregnate palladium salt in the autoclave, seal the autoclave, and then pass in argon and hydrogen. , Where the hydrogen pressure is 5.0~8.0Mpa; the hydrogenat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com