A self-doped conjugated phenolic amine hole transport material and its preparation and application
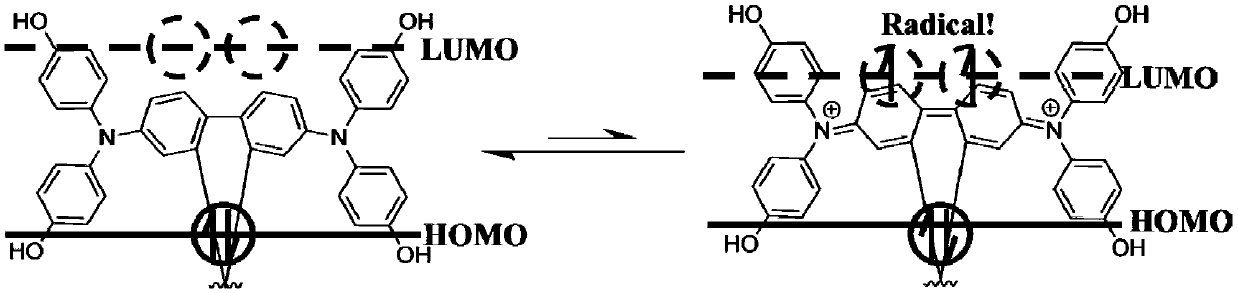
A technology of hole transport material and conjugated phenolic amine, which is applied in the field of self-doping conjugated phenolic amine hole transport material and its preparation and application, can solve the problems of cost increase and achieve the goal of preventing interface erosion and high conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
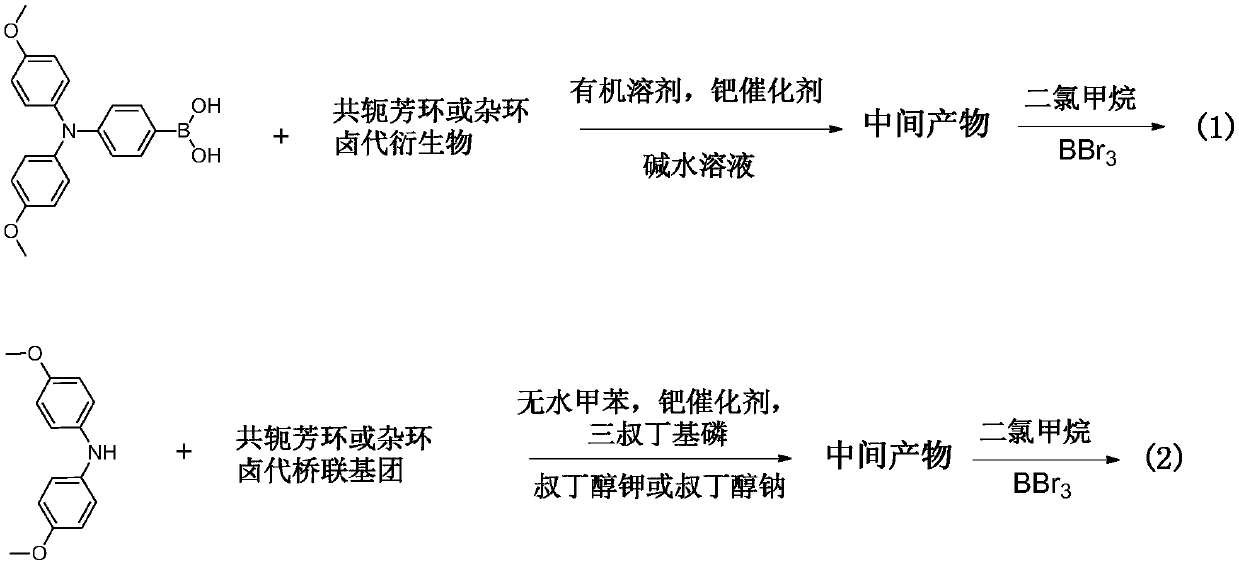
[0046] 4,7-dibromo-benzothiadiazole (0.59g, 2.00mmol), 4,4-dimethoxydiphenyl-4-boronic acid-aniline (1.75g, 5.00mmol), tetrakistriphenylphosphine Palladium (230mg, 0.2mmol) was added to a mixed solvent of anhydrous toluene (20mL) and ethanol (10mL), and 5mL of 2M sodium carbonate aqueous solution was added under the protection of nitrogen, and the reaction was refluxed for 8h. The reaction product was separated and purified to obtain intermediate Product 1.45g, yield 98%. Dissolve the above product (0.5 g) in 15 ml of dichloromethane solution, exhaust nitrogen gas for 20 min, add 6 mL of boron tribromide at -78 ° C, gradually raise to room temperature, and react for 72 h, add an appropriate amount of ice water to quench excess boron tribromide, extract the product three times with 30mL ethyl acetate, collect the organic phase, remove the solvent under reduced pressure, and separate with a silica gel column, using petroleum ether / ethyl acetate=1:1 (v / v) as eluent , and then wa...
Embodiment 2
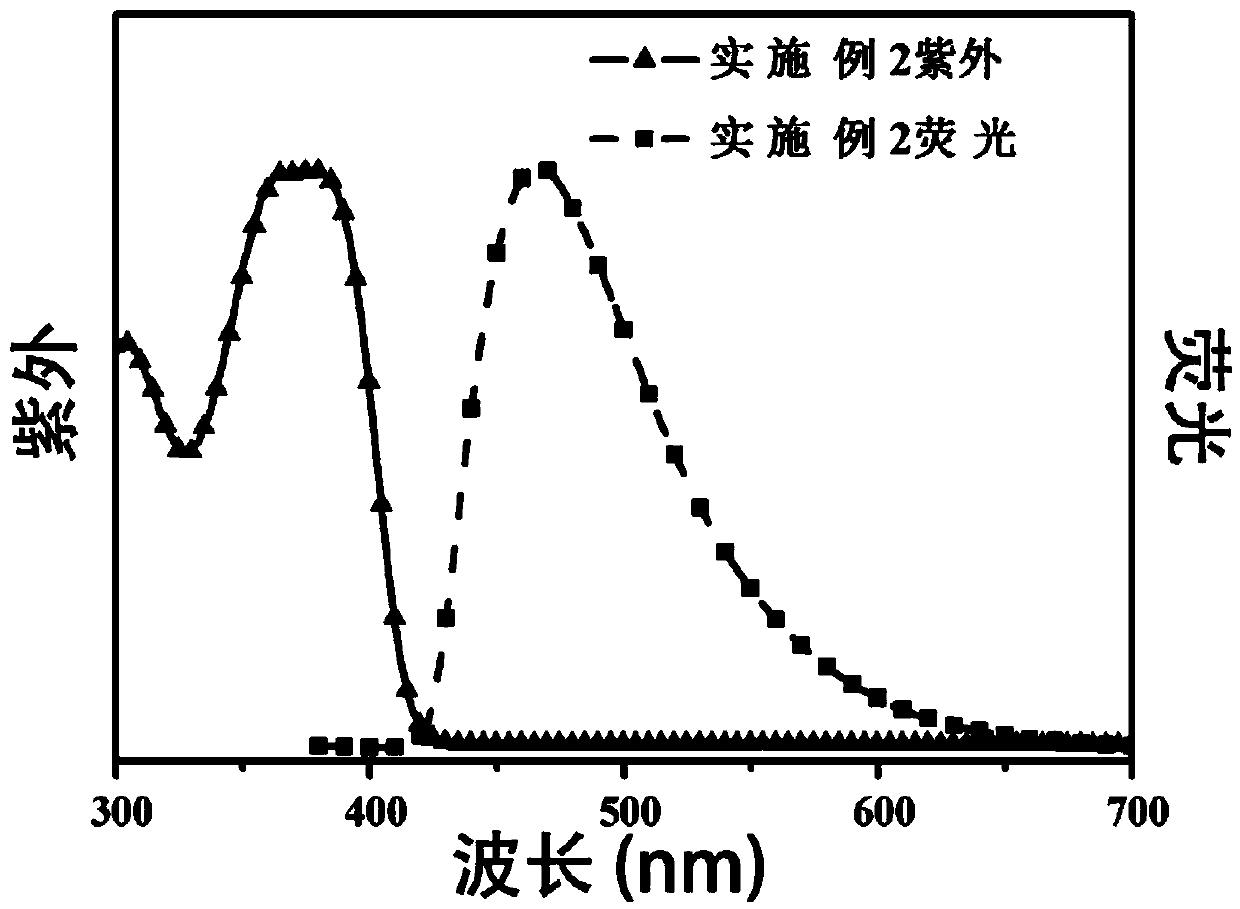
[0049] Dissolve Spiro-OMeTAD (0.3g) in 10ml of dichloromethane solution, exhaust nitrogen for 20min, add 3mL of boron tribromide at -78°C, gradually raise to room temperature, and react for 48h, then add appropriate amount of methanol to quench excess boron tribromide, extract the product three times with 30mL ethyl acetate, collect the organic phase, remove the solvent under reduced pressure, and separate with a silica gel column, using petroleum ether / ethyl acetate=1:3 (v / v) as eluent , the crude product was obtained, and then washed 3 times with petroleum ether to obtain 0.25 g of gray-green powder, with a yield of 80%. 1 H-NMR (600MHz, DMSO-d 6 )δ9.40-9.18(s,8H),7.48-7.32(d,J=12Hz,4H),6.83-6.70(m,16H),6.68-6.61(m,16H),6.61-6.57(dd,J =1.8,6Hz,4H),6.20-6.06(s,4H).C 73 h 52 N 4 NaO 4 [M + Na + ] + 1135.3677, found 1135.3669. The structural formula of the product obtained is as follows:
[0050]
[0051] The resulting product has a solubility greater than 200mg / mL i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com