A star-shaped aromatized inorganic acid radical semiconductor material and its preparation and application
An inorganic acid and free radical technology, which is applied in the manufacture of semiconductor/solid-state devices, semiconductor devices, and the preparation of imino compounds, can solve the problems of difficulty in designing organic small molecule semiconductor materials, high reactivity, etc. Conductivity, the effect of stabilizing free radical signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
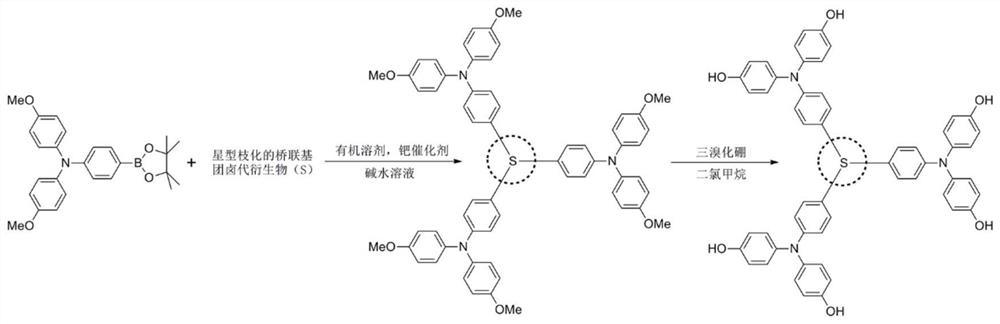
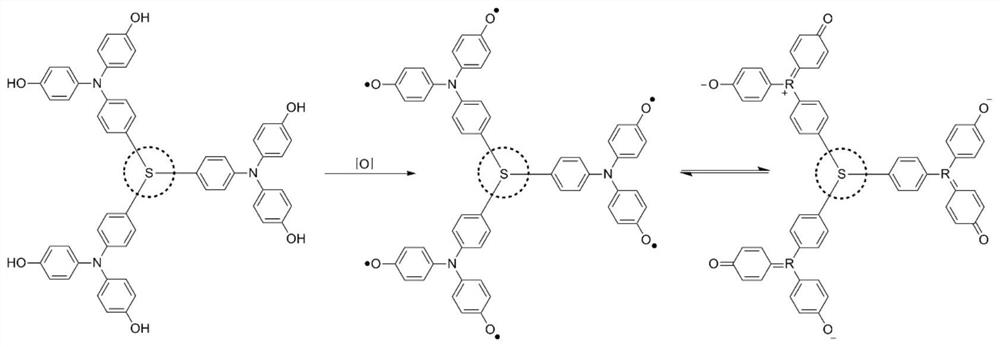
[0040] Combine 4-boronate-4',4'-dimethoxytriphenylamine (754mg, 1.75mmol) with tris(4-bromophenyl)amine (241mg, 0.5mmol), tetrakistriphenylphosphine palladium (58mg, 0.05mmol), was added to a mixed solvent of 30ml of toluene and 10ml of ethanol, 3ml of 2M sodium carbonate solution was added under nitrogen protection, and reflux reaction was carried out at 110°C for 12h. After separation and purification, 324mg of the reaction intermediate product was obtained, with a yield of 56.2 %. Dissolve 230 mg of the above product in 15 ml of dichloromethane, and add 3 ml of 1M concentration of BBr at -78°C in a nitrogen atmosphere 3 , and then moved to room temperature for 12h, quenched with methanol. The reaction product was further oxidized in air after separation and purification to obtain 184 mg of the product with a yield of 86%.
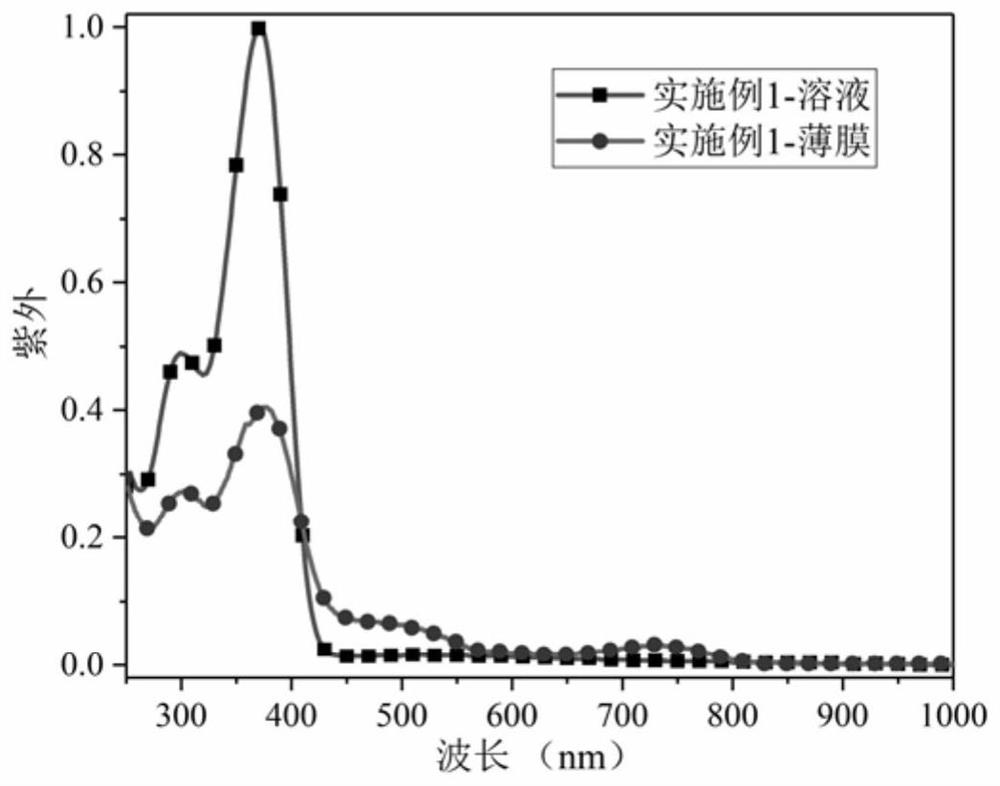
[0041] The ultraviolet-visible light absorption spectrum and electron paramagnetic resonance spectrum of the product obtained in this embodiment are s...
Embodiment 2
[0050] 4-boronate-4',4'-dimethoxytriphenylamine (754mg, 3.5mmol) and 2,4,6-tris-(4-bromophenyl)-[1,3,5]tri Add oxazine (273mg, 0.5mmol) and tetrakistriphenylphosphine palladium (58mg, 0.05mmol) into a mixed solvent of 30ml of toluene and 10ml of ethanol, add 3ml of 2M sodium carbonate solution under nitrogen protection, and reflux at 110°C After 12 hours of reaction, 373 mg of a reaction intermediate was obtained after separation and purification, with a yield of 62.0%. Dissolve 243 mg of the above product in 15 ml of dichloromethane, and add 3 ml of 1M concentration of BBr at -78°C under a nitrogen atmosphere 3 , and then moved to room temperature to react for 12h, and the reaction product was further oxidized in air after separation and purification to obtain 198mg of the product with a yield of 87.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com