Method used for preparing epsilon-hexanolactone
A technology of caprolactone and cyclohexanone, which is applied in the field of preparation of organic compounds, can solve the problems of high cost and low efficiency of pro-oxidant benzaldehyde, achieve high industrial value, facilitate separation and purification, and improve economic feasibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
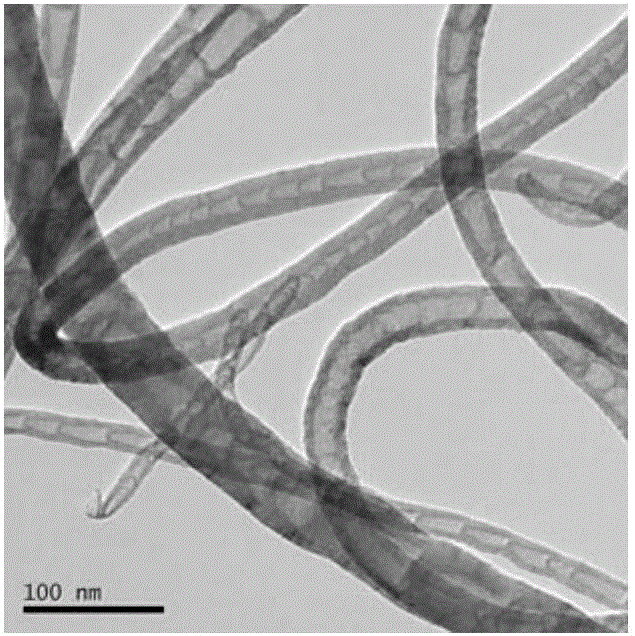
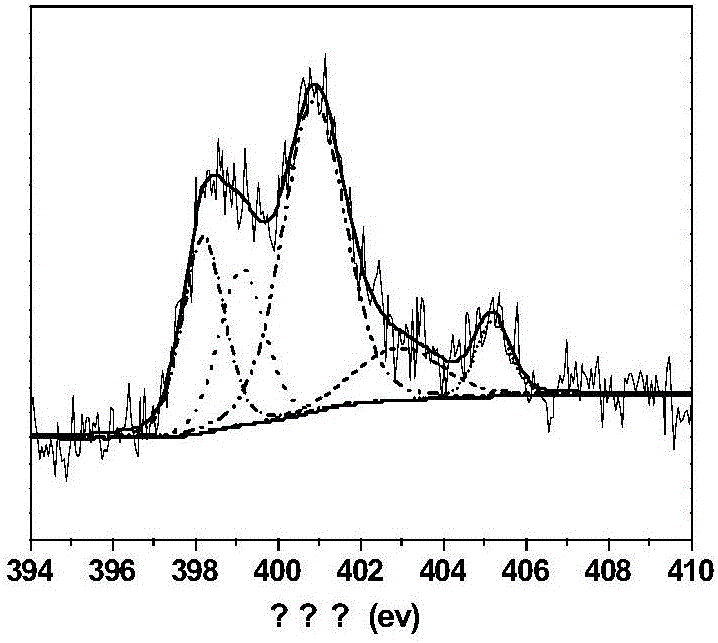
Image
Examples
Embodiment 1~5
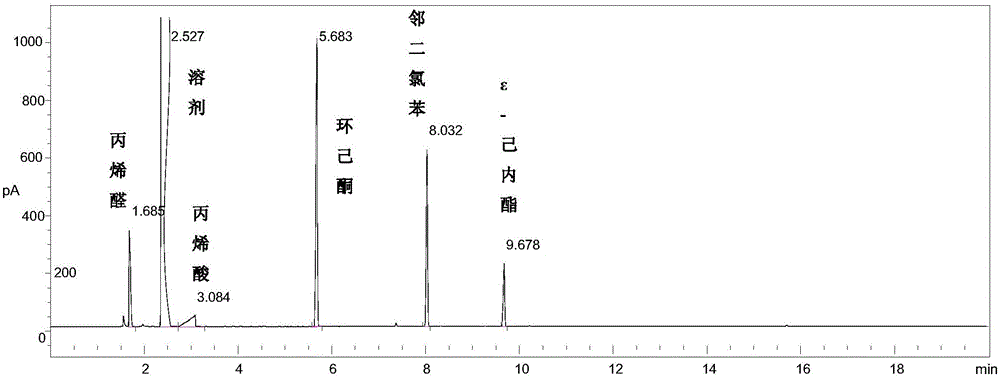
[0026] Add 25ml 1,2-dichloroethane, 2.6g o-dichlorobenzene (internal standard), 4.75g cyclohexanone, 2.69g acrolein and 100mg nitrogen-doped carbon nanotubes (N content is 4.34at%) Stir and heat to the high-pressure reactor to the temperature shown in Table 1, feed oxygen, start timing, and maintain the pressure at 1 MPa during the reaction. After reacting for 4 hours, stop timing, cool the reactor to room temperature, and filter the liquid-solid phase mixture to obtain a solid catalyst and a liquid phase mixture containing unreacted reactants and reaction products. The liquid phase mixture was detected and analyzed by gas chromatography (GC). The gas chromatogram of the reaction solution after the reaction of embodiment 3 is as follows image 3 shown. The GC detection results are shown in Table 1 (the influence of reaction temperature on the Baeyer-Villiger oxidation reaction of cyclohexanone).
[0027] Table 1
[0028] Example 1 2 3 4 5 Reaction tempe...
Embodiment 6~12
[0031] Add 25ml 1,2-dichloroethane, 2.6g o-dichlorobenzene (internal standard), 4.75g cyclohexanone, 2.69g acrolein and 100mg nitrogen-doped carbon nanotubes (N content is 4.34at%) Stir and heat to 80°C in a high-pressure reactor, feed oxygen, start timing, and maintain the pressure at 1 MPa during the reaction. After reacting to the time shown in Table 2, stop timing, the reactor is cooled to room temperature, and the liquid-solid phase mixture is filtered to obtain a solid catalyst and a liquid phase mixture containing unreacted reactants and reaction products. The liquid phase mixture was detected and analyzed by gas chromatography (GC). The GC detection results are shown in Table 2 (the influence of reaction time on the Baeyer-Villiger oxidation reaction of cyclohexanone).
[0032] Table 2
[0033]
[0034]
[0035] Analysis of the data in Table 2 shows that the conversion rate of cyclohexanone increases with the prolongation of time, the efficiency of acrolein dec...
Embodiment 13~16
[0037]Add 25ml 1,2-dichloroethane, 2.6g o-dichlorobenzene (internal standard), 4.75g cyclohexanone, 2.69g acrolein and 100mg nitrogen-doped carbon nanotubes (N content is 4.34at%) Stir and heat to 80°C in a high-pressure reactor, feed oxygen, start timing, and maintain the pressure as shown in Table 3 during the reaction, stop timing after reacting for 4 hours, cool the reactor to room temperature, and filter the liquid and solid phase mixture to obtain a solid catalyst and a liquid phase mixture containing unreacted reactants and reaction products. The liquid phase mixture was detected and analyzed by gas chromatography (GC). The GC detection results are shown in Table 3 (the influence of reaction pressure on the Baeyer-Villiger oxidation reaction of cyclohexanone).
[0038] table 3
[0039] Example 13 14 3 15 16 Reaction pressure (MPa) 0.1 0.5 1 1.5 2 Cyclohexanone conversion rate (%) 13 19 22 24 27 ε-caprolactone selectivity (%) 88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com