Antibody-coupled bionic immune magnetic sphere and preparation method thereof
A bionic immune and antibody technology, applied in the field of nanomaterials, can solve problems such as unfavorable CTC analysis and non-specific adsorption of white blood cells, and achieve the effects of good cell activity, high recognition efficiency, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
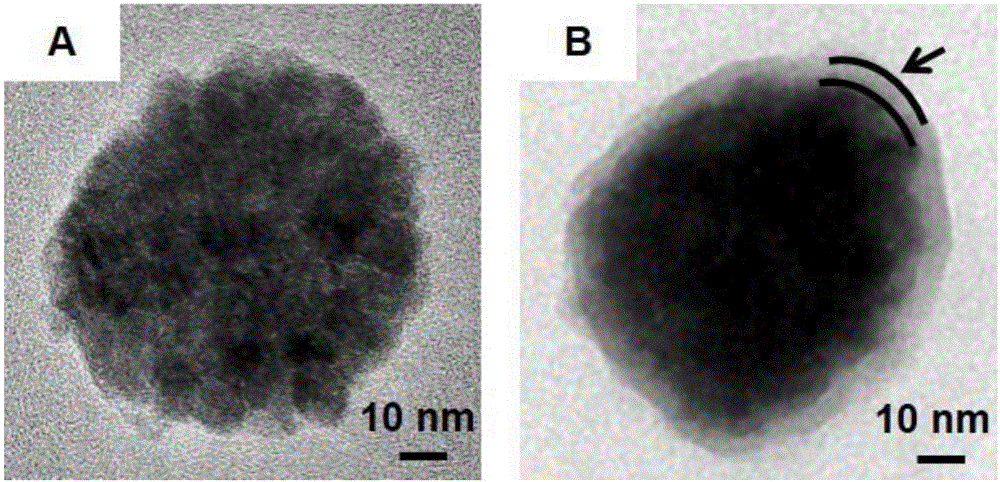
[0089] The preparation of embodiment 1MNC
[0090] (1) Put DEG in the environment of Ar gas, after the oxygen is removed completely, dissolve the solid NaOH into the DEG, heat at the same time, raise the temperature to 120°C, stop heating until the solid NaOH is completely dissolved, the whole preparation process uses Protected by Ar gas, a light yellow solution was prepared, which was a NaOH storage solution with a concentration of 1g / mL, and kept at 70°C for future use;
[0091] (2) FeSO 4 ·7H 2 O. PEI and DEG are mixed evenly, then vacuumed, and then filled with Ar gas protection; heat up to 160 ° C, add the NaOH storage solution obtained in step 1 (1), until the molar concentration of NaOH in the solution is 0.03mol / L, about 3min Afterwards, the color of the solution turned black, and then the temperature was raised to 220° C., continued to condense and reflux for 1 hour, and the reaction was completed, and a reaction solution containing positively charged MNC was obtain...
Embodiment 2
[0103] Preparation of Example 2M
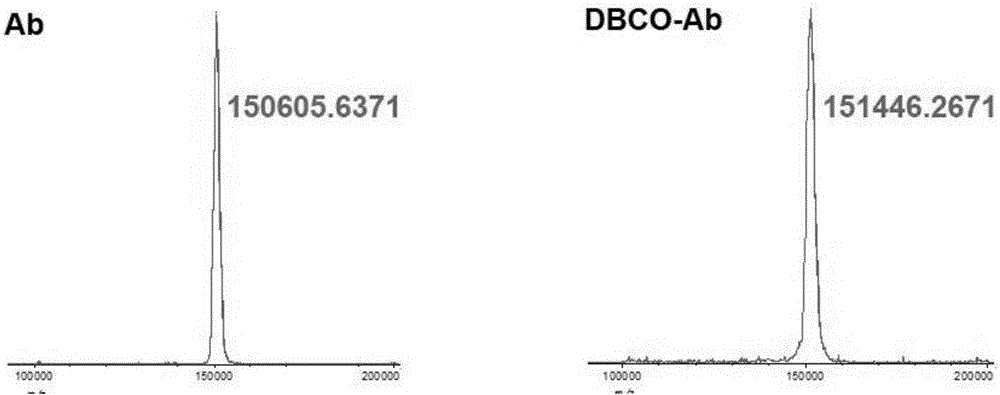
[0104] Place the leukocyte J774A.1 cell line at 37°C, CO 2 In a constant temperature incubator with a concentration of 5%, culture with complete cell culture medium for 24 hours, respectively replace it with complete cell culture medium containing 0.1 mM azidecholine for 24 hours, collect white blood cells, and discard the medium.
[0105] Resuspend the collected leukocytes with Hepes B buffer at pH 7.6, add 10 μL / mL of protease inhibitors, break the cells intermittently with a homogenizer at gear 2, break for 2 minutes, stop for 1 minute, repeat 10 times, and then centrifuge at 1000 r / min 3min, collect the supernatant, resuspend the pellet with Hepes B buffer added with 10μL / mL protease inhibitor, break the cells with a homogenizer, break for 2min, stop for 1min, repeat 10 times, then centrifuge at 1000r / min for 3min, collect the supernatant Supernatant and supernatant were also suspended in Hepes B buffer containing protease inhibitors, an...
Embodiment 3
[0116] Preparation of Example 3M
[0117] Place the leukocyte J774A.1 cell line at 37°C, CO 2 In a constant temperature incubator with a concentration of 5%, culture with complete cell culture medium for 20 hours, replace it with cell culture complete medium containing 0.1 mM azidecholine and continue to cultivate for 22 hours, collect white blood cells, discard the medium, and prepare the rest The method and control experiment are the same as in Example 2.
[0118] The prepared M-solution and M-solution were tested and characterized, and the results were similar to those in Example 2. Preparation of Example 4M
[0119] Place the leukocyte J774A.1 cell line at 37°C, CO 2 In a constant temperature incubator with a concentration of 5%, culture with complete cell culture medium for 28 hours, replace it with cell culture complete medium containing 0.1 mM azidecholine and continue to cultivate for 28 hours, collect white blood cells, discard the medium, and prepare the rest The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com