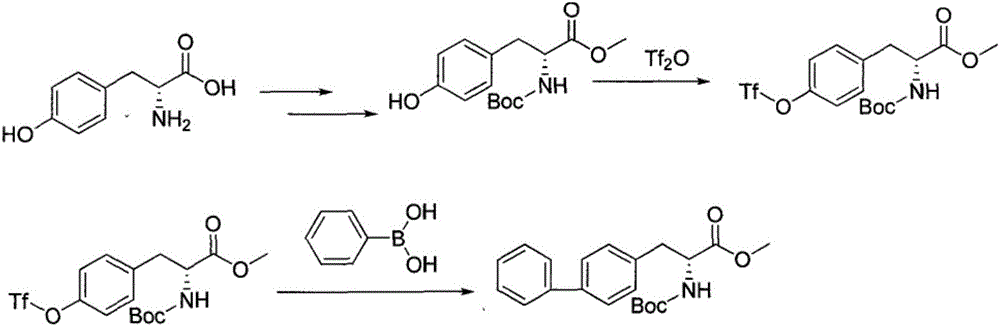
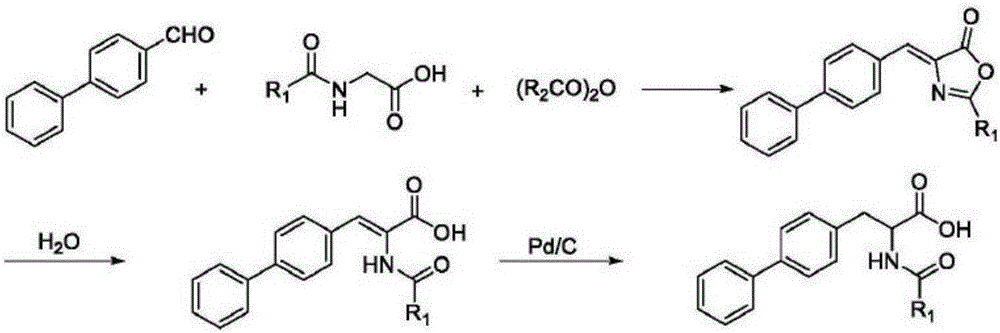
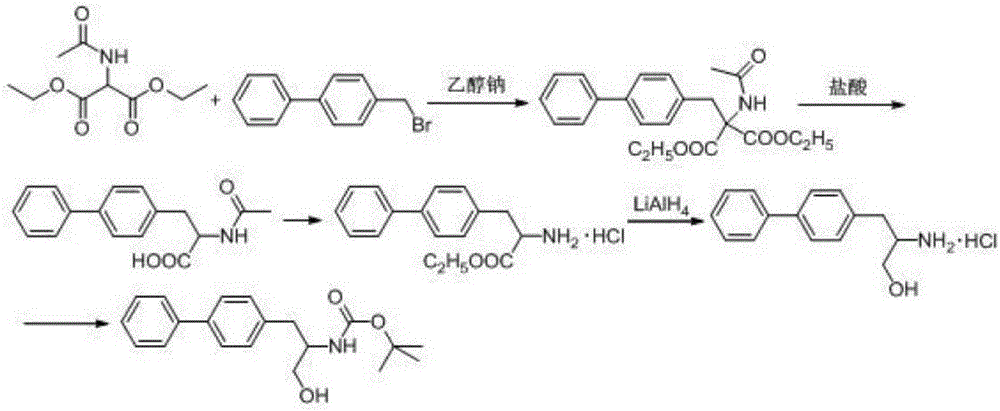
Method for synthesizing D-biphenyl alanine
A technology of biphenylalanine and diethyl acetamidomalonate is applied in the field of synthesizing D-biphenylalanine, which can solve the problem of high chirality that cannot be solved by chemical methods, and is unfavorable for industrialized production and synthesis Problems such as low yield, to achieve the effect of easy industrialized large-scale production, simple environmental protection, and high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Add 1200kg of ethanol to the reaction kettle, start stirring, add 200kg of diethyl acetamidomalonate, and then add 28kg of sodium metal. Then add 150 kg of 4-bromomethyl biphenyl, keep the temperature at 50° C. for 2 hours, cool down and crystallize to obtain 320 kg of the condensate shown in Formula 1.
[0032] 2) Add 1100kg of 0.5mol / L hydrochloric acid into the reaction kettle, start stirring, add 320kg of the condensate shown in formula 1, stir at 25-30°C for 2 hours, cool down and crystallize to obtain N-acetyl-DL-biphenylalanine Acid 150kg.
[0033] 3) Add 1800kg of water to the reaction kettle, add 150kg of N-acetyl-biphenylalanine, adjust the pH to 7.6 with ammonia water, raise the temperature to 30°C, add L-aminoacylase and L-alanine for racemization Enzymes, a total of 0.15g, wherein the enzyme activity ratio of L-aminoacylase and L-alanine racemase is 1:2.1, reacted for 10 hours, a large amount of D-biphenylalanine crystallized out, centrifuged , rinsed ...
Embodiment 2
[0036] 1) Add 2500kg of ethanol to the reaction kettle, start stirring, add 416kg of diethyl acetamidomalonate, and then add 62.5kg of sodium metal. Then add 315 kg of 4-bromomethylbiphenyl, and keep the temperature at 50° C. for 2 hours. Cooling and crystallization gave 669 kg of the condensate shown in Formula 1.
[0037] 2) Add 2300kg of 0.5mol / L hydrochloric acid into the reaction kettle, start stirring, add 669kg of the condensate shown in formula 1 obtained in step 1, stir at 25-30°C for 2 hours, cool down and crystallize to obtain N-acetyl-DL-linked Phenylalanine 314kg.
[0038] 3) Add 3780kg of water to the reaction kettle, add 314kg of N-acetyl-biphenylalanine, adjust the pH to 7.6 with ammonia water, raise the temperature to 30°C, add L-aminoacylase and L-alanine for racemization Enzymes, a total of 0.471g, wherein, the enzyme activity ratio of L-aminoacylase and L-alanine racemase is 2.5, reacted for 10 hours, a large amount of D-biphenylalanine crystallized out, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com