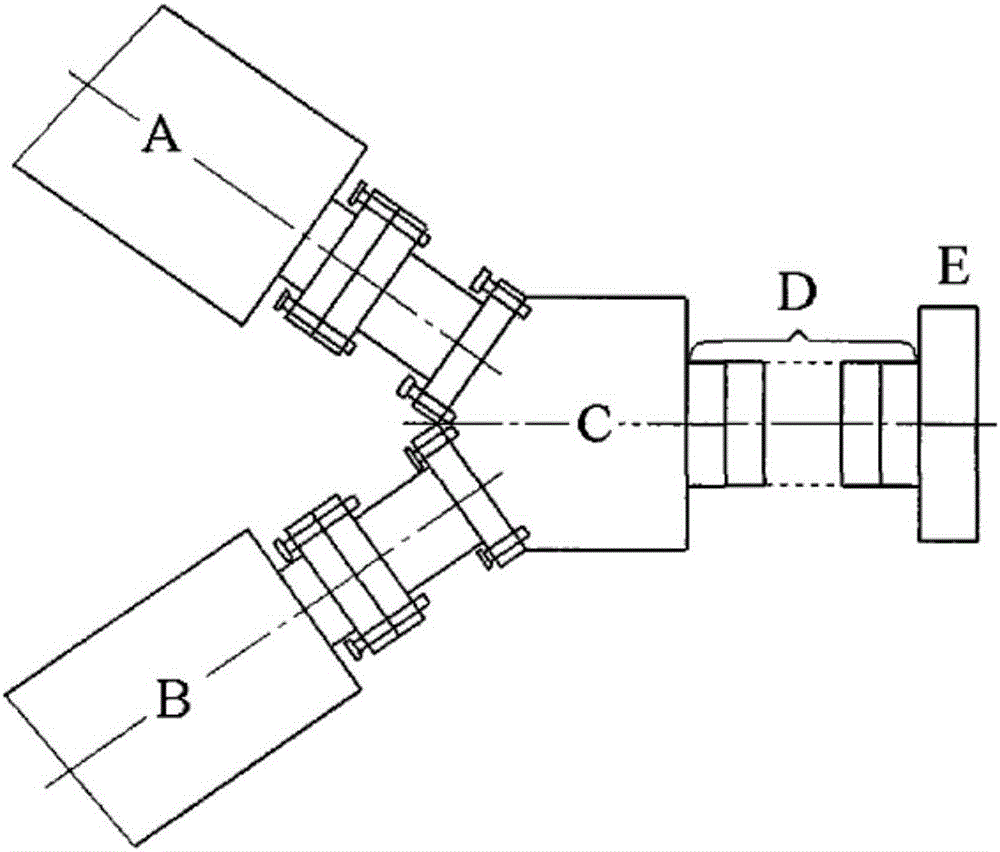
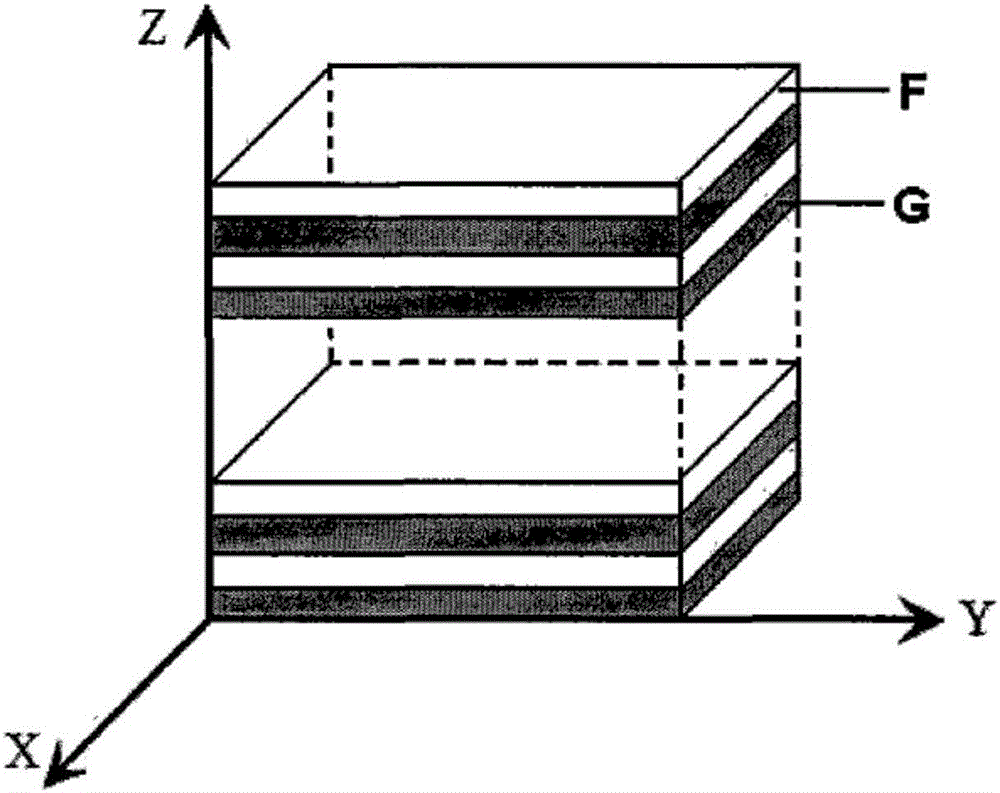
Method for preparing alternate lamellar bio-degraded polymer drug controlled-release composite
A polymer material and biodegradation technology, which is applied in the field of preparation of alternate layered biodegradable polymer drug slow-release materials, can solve the problems of low drug efficacy, complex process, poor preparation stability, etc. Good compatibility and the effect of refining the size of the dispersed phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In the first step, the polymer-based drug immediate-release layer (hereinafter referred to as the immediate-release layer) uses polycaprolactone as the matrix of the immediate-release layer (molecular weight: 80,000), and polyethylene oxide as the dispersed phase of the immediate-release layer (molecular weight: 100,000), and The ingredients are 60%:40% by weight; metoprolol tartrate is the drug of the immediate release layer, and the ingredients are formulated according to 10% of the total weight of the polymer of the immediate release layer. The above-mentioned polymer and drug were mixed in a high-speed mixer for 5 minutes at a speed of 100 rpm to obtain a polymer-based drug immediate-release layer material.
[0050] The polymer-based drug slow-release layer (hereinafter referred to as the slow-release layer) selects polycaprolactone as the slow-release layer matrix (molecular weight is 80000), and metoprolol tartrate is the slow-release layer drug, and the percentage...
Embodiment 2
[0055] The polymer-based drug immediate-release layer (hereinafter referred to as the immediate-release layer) uses polybutylene succinate as the matrix of the immediate-release layer (molecular weight is 100,000); polyethylene oxide is the dispersed phase of the immediate-release layer (molecular weight is 200,000); and Percentage by weight 80%: 20% batching; Ibuprofen is an immediate-release layer medicine, and is batched according to 35% of the total weight of the immediate-release layer macromolecule. The above-mentioned polymer and drug were mixed in a high-speed mixer for 5 minutes at a speed of 100 rpm to obtain a polymer-based drug immediate-release layer material.
[0056] The polymer-based drug slow-release layer (hereinafter referred to as the slow-release layer) uses polycaprolactone as the matrix of the slow-release layer (molecular weight is 50000); polyethylene glycol is the dispersed phase of the slow-release layer (molecular weight is 6000); 95%: 5% batching; ...
Embodiment 3
[0061] In the first step, the polymer-based drug immediate-release layer (hereinafter referred to as the immediate-release layer) uses ethyl cellulose as the matrix of the immediate-release layer (molecular weight: 130,000), and polyethylene glycol as the dispersed phase of the immediate-release layer (molecular weight: 6,000). And with weight percentage 75%: 25% batching; Hydrochlorothiazide is the immediate-release layer medicine, carries out batching according to 30% of the macromolecule gross weight of the quick-release layer. The above-mentioned polymer and drug were mixed in a high-speed mixer for 5 minutes at a speed of 100 rpm to obtain a polymer-based drug immediate-release layer material.
[0062] The polymer-based drug sustained-release layer (hereinafter referred to as the sustained-release layer) selects ethyl cellulose as the matrix of the sustained-release layer (molecular weight is 130000), and metoprolol tartrate as the drug, 91% by weight: 9% in high blender ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com