Preparation of hollow Mn2O3 micro-spheres and application method thereof in lithium battery
An application method, the technology of microspheres, applied in the direction of lithium batteries, battery electrodes, non-aqueous electrolyte batteries, etc., can solve the problems of complex preparation methods of synthetic hollow structures, unfavorable commercial promotion, high cost, etc., to achieve commercial promotion, Good cycle stability, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of Mn-BTC microspheres
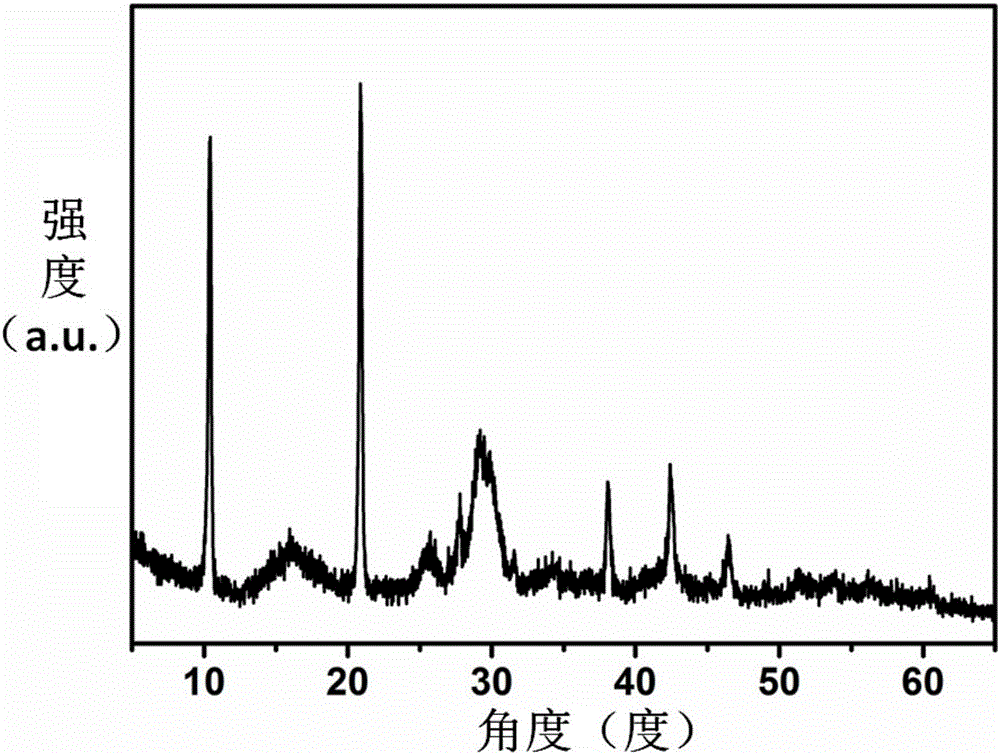
[0029] At room temperature at 25°C, drop a mixed solution of 10 mL of ethanol containing 90 mg of trimesic acid and water (volume ratio of 1:1) into 10 mL of ethanol containing 49 mg of manganese acetate tetrahydrate and 0.3 g of polyvinylpyrrolidone and In a mixed solution of water (volume ratio 1:1), stir evenly, let stand for 24 hours, and centrifuge to obtain the precursor Mn-BTC (complex formed by manganese and trimesic acid) microspheres. X-ray diffraction of the precursor Figure such as figure 1 As shown, the appearance characteristics are as figure 2 shown.
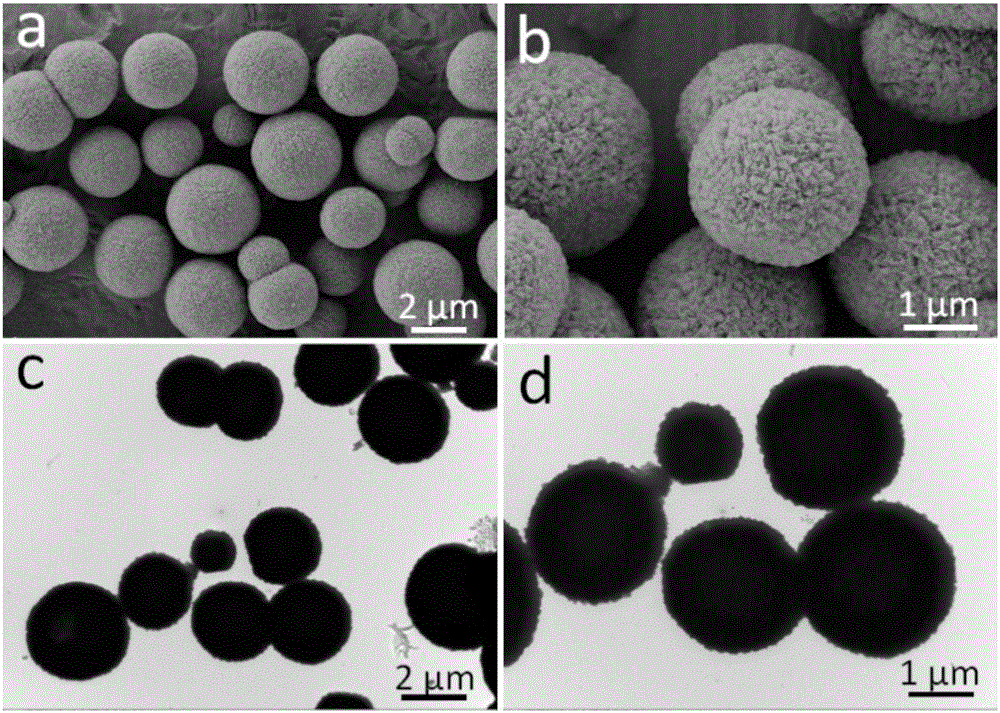
[0030] figure 2 are scanning electron microscope (SEM) and transmission electron microscope (TEM) pictures of precursors at different magnifications. It can be seen from the figure that the precursor exists in the form of microspheres with a uniform shape, a particle size of about 2 μm, and a solid structure.
Embodiment 2
[0031] Example 2: Preparation of hollow Mn 2 o 3 microsphere
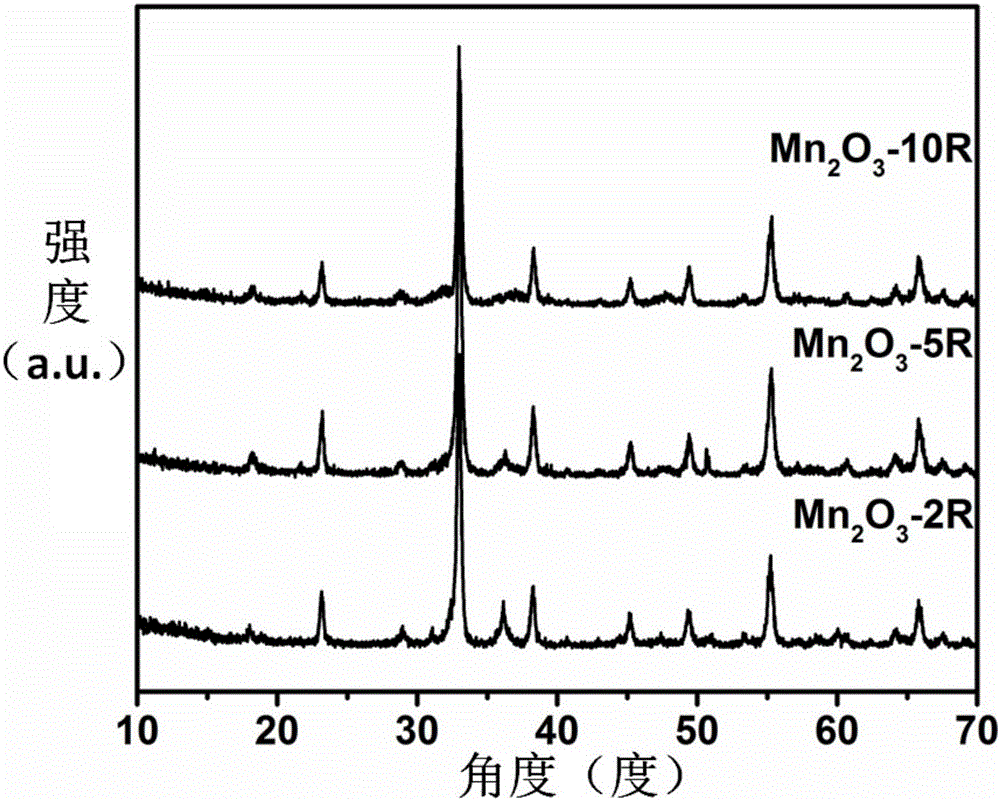
[0032] The precursor Mn-BTC microspheres obtained in Implementation 1 were placed in a muffle furnace, and the temperature was raised to 450° C. at a rate of 10° C. / min in an air atmosphere. Then calcined at this temperature for 2 hours, after natural cooling to room temperature, the hollow Mn 2 o 3 Microspheres (Mn 2 o 3 -10R). The chemical composition of the calcined product can be determined by X-ray diffraction to determine, such as image 3 shown. From image 3 It can be seen that the X-ray diffraction peak of the obtained product is consistent with the standard JCPDS card No.41-1442, indicating that the obtained sample is Mn 2 o 3 . In addition, through the scanning electron microscope and transmission electron microscope photographs of different magnifications of the product, Mn 2 o 3 The appearance characteristics of -10R were analyzed by nitrogen adsorption experiment. Such as Figure 4 As s...
Embodiment 3
[0033] Embodiment 3: the influence of heating rate on product
[0034] As with other conditions in Example 2, the precursor Mn-BTC microspheres were heated up to 450°C at a rate of 5°C / min and 2°C / min, respectively, and then calcined at this temperature for 2 hours to obtain the black product Mn 2 o 3 -5R and Mn 2 o 3 -2R. and the samples calcined at a rate of 10°C / min to 450°C (Mn 2 o 3 -10R), as in image 3 As shown, all obtained are Mn 2 o 3 . Figure 5 Corresponding to Mn in 2 o 3 -5R and Mn 2 o 3 -SEM pictures and TEM pictures at different magnifications of 2R. From Figure 5 It can be seen from the figure that when the heating rate is reduced from 5°C / min to 2°C / min, the product still maintains a spherical appearance, but the degree of damage of the hollow spheres is increasing. And, Mn 2 o 3 The hollow sphere structure of -10R is relatively Mn 2 o 3 -5R and Mn 2 o 3 -2R is relatively complete. Therefore, as the heating rate decreases, the degree of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com