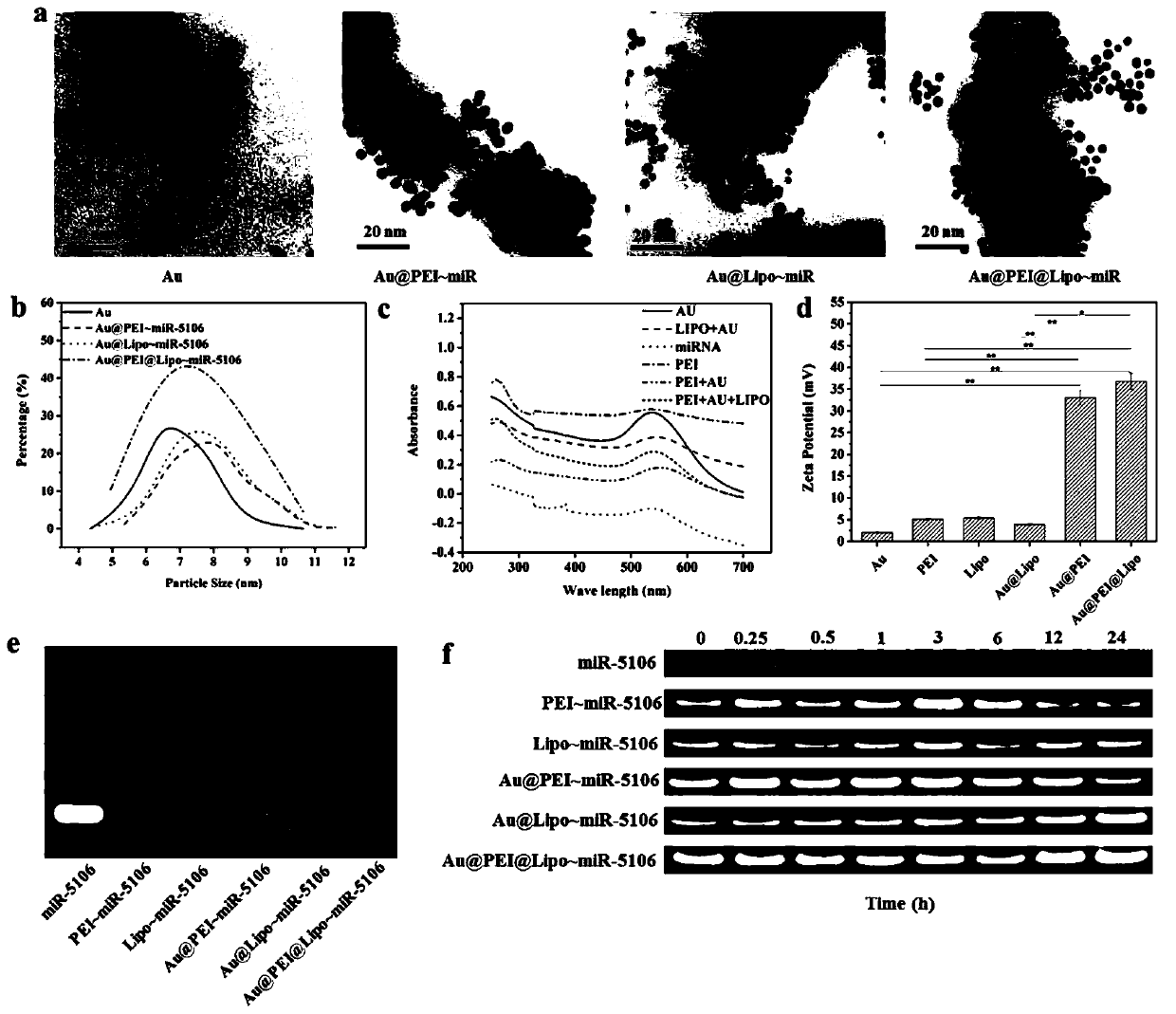
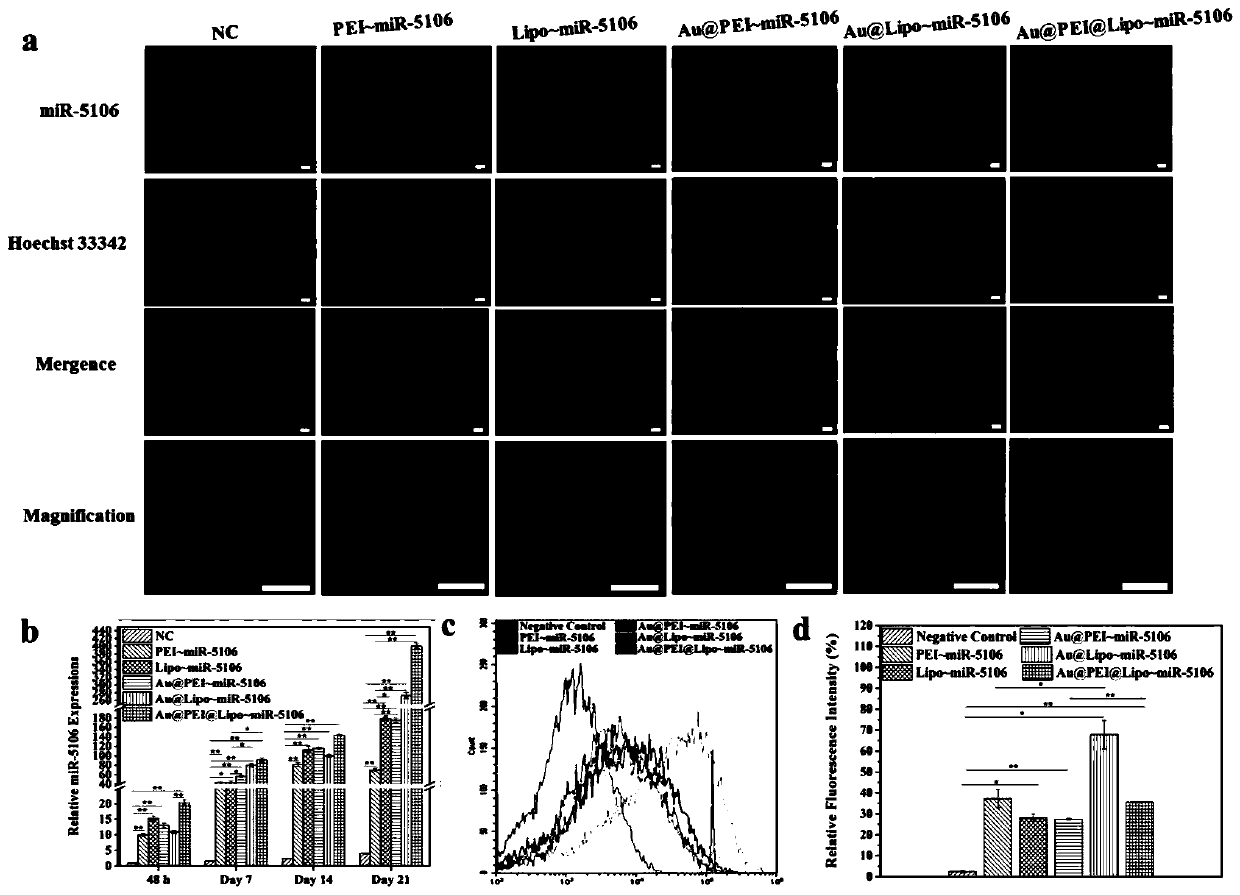
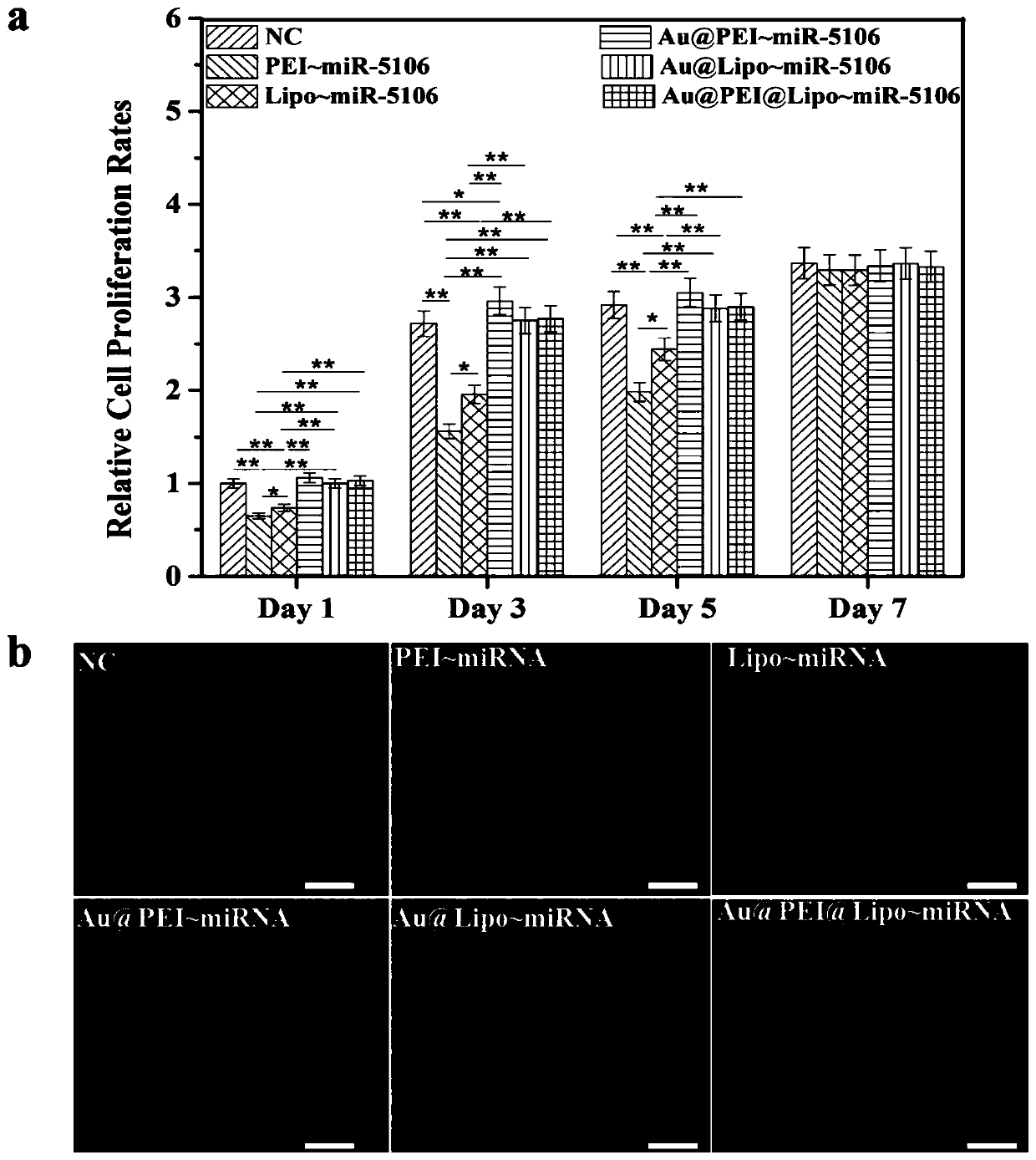
A miRNA delivery carrier based on ultra-small gold nanoparticles and its preparation method and application
A nanoparticle and delivery carrier technology, applied in biochemical equipment and methods, recombinant DNA technology, medical preparations of non-active ingredients, etc., can solve the problems of serious side effects, high reaction temperature, long-term toxicity, etc., to reduce toxicity , high controllability, and the effect of promoting osteogenic differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Preparation of ultra-small gold nanoparticles: add chloroauric acid and citric acid to deionized water according to the molar ratio of 1:0.9, stir vigorously and quickly add the freshly prepared sodium borohydride solution dropwise to obtain the reaction solution, continue to stir the reaction After 5 hours, the reaction system was centrifuged and resuspended in water to obtain citric acid-capped ultra-small gold nanoparticles with a size of about 8 nm. Wherein the sodium borohydride in the sodium borohydride solution that adds and the mol ratio of chloroauric acid are 22.5:1, and the concentration of chloroauric acid in the reaction solution is 4mmol / L, and the concentration of citric acid is 3.6mmol / L, sodium borohydride The concentration is 0.09mol / L.
[0042] 2) Modification of ultra-small gold nanoparticles: 0.04 mmol of ultra-small gold nanoparticles prepared in step 1) were dissolved in 15 mL of sodium hydroxide solution at pH=10, and 0.036 mmol of 16-mercapto...
Embodiment 2
[0046] 1) Preparation of ultra-small gold nanoparticles: Add chloroauric acid and citric acid into deionized water according to the molar ratio of 0.2:0.2, stir vigorously and quickly add the freshly prepared sodium borohydride solution dropwise to obtain the reaction solution, and continue to stir the reaction After 6 hours of reaction, the reaction system was centrifuged and resuspended in water to obtain citric acid-capped ultra-small gold nanoparticles with a size of about 9 nm. Wherein the sodium borohydride in the sodium borohydride solution that adds and the mol ratio of chloroauric acid are 12:0.6, and the concentration of chloroauric acid in the reaction solution is 2mmol / L, and the concentration of citric acid is 2mmol / L, the sodium borohydride The concentration is 0.04mol / L.
[0047] 2) Modification of ultra-small gold nanoparticles: 0.01 mmol of ultra-small gold nanoparticles prepared in step 1) were dissolved in 20 mL of sodium hydroxide solution at pH=9, and 0.01...
Embodiment 3
[0051] 1) Preparation of ultra-small gold nanoparticles: Add chloroauric acid and citric acid into deionized water according to the molar ratio of 0.625:1, stir vigorously and quickly add the freshly prepared sodium borohydride solution dropwise to obtain the reaction solution, continue to stir the reaction After 8 hours of reaction, the reaction system was centrifuged and resuspended in water to obtain citric acid-capped ultra-small gold nanoparticles with a size of about 10 nm. Wherein the sodium borohydride in the sodium borohydride solution that adds and the mol ratio of chloroauric acid are 30:1.25, and the concentration of chloroauric acid in the reaction solution is 5mmol / L, and the concentration of citric acid is 8mmol / L, the sodium borohydride The concentration is 0.12mol / L.
[0052] 2) Modification of ultra-small gold nanoparticles: 0.02 mmol of ultra-small gold nanoparticles prepared in step 1) were dissolved in 25 mL of sodium hydroxide solution at pH=11, and 0.02 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com