Haploid maize transformation
A haploid and double haploid technology, applied in the field of haploid maize transformation, can solve the problem that no one has explored site specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0271] Example 1: Generation of microspore-derived haploid callus
[0272] Maize genotype 139 / 39-05 (U.S. Patent No. 5,306,864) was harvested from greenhouse-grown maize plants (approximately 5-6 weeks old) when the microspores were in the early binucleate stage of development (anthers approximately 3 mm long, bright, lustrous yellow) The pre-emergent tassel (Pre-emergent tassel). The tassels were wrapped in wet paper towels and aluminum foil and placed in an incubator set at 8°C for 7-14 days. Sterilized on surfaces (at 0.08% v / v Chlorox TM 15 minutes, followed by rinsing with sterile water), the anthers were aseptically separated, and placed in a liquid "anther culture medium" (N6 salt and Vitamins, 60 g / L sucrose, 5 g / L activated carbon, 500 mg / L casein hydrolyzate, 0.1 mg / L TIBA, adjusted to pH 5.8) on the surface and incubated at 28°C in the dark. Microspore-derived embryo-like structures that appeared between 14-28 days were transferred to "callus medium" (MS salts an...
Embodiment 2
[0274] Example 2: Ploidy determination of callus
[0275] In order to measure the cell ploidy level, 1 g of the callus prepared in Example 1 was transferred to sterile Petri TM Dishes (Fisher Scientific, St. Louis, MO). Pass through ice-cold Gailbraith buffer (0.01M MgSO) filtered in 1-2mL 4 , 0.005M KCl, 0.0005M HEPES, 1mg / mL DTT) and "MMG Medium" (4mM MES [pH 6.0], 0.6M Mannitol, 15mM MgCl 2 ) and 0.25% Triton X-100 TM The callus was minced with a single-edged razor blade in the presence of the nucleus to release it. Rinse the Petri with another 2 mL of buffer TM dish, which was combined with the initial nuclear extract to give a final slurry volume of approximately 5 mL. The crude nuclear extract was then gently homogenized by transferring to a glass tissue homogenizer, pumping the plunger up and down several times. Then, the homogenate was filtered through a tea strainer, 40 μm Steri-flip TM (Millipore; Billerica, Massachusetts, USA) the resulting filtrate was aspir...
Embodiment 3
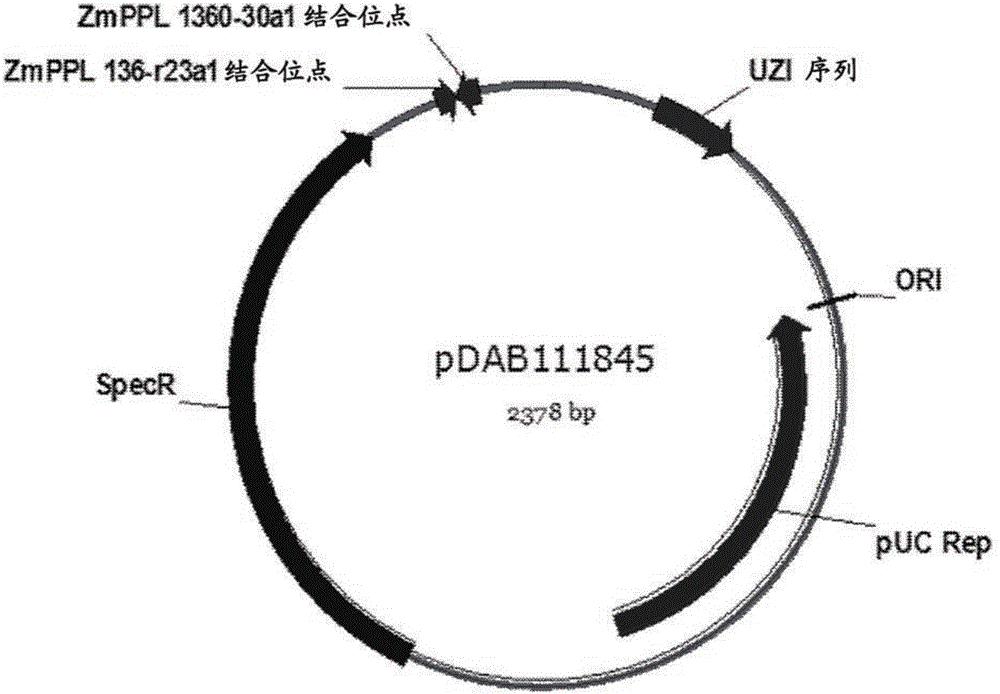
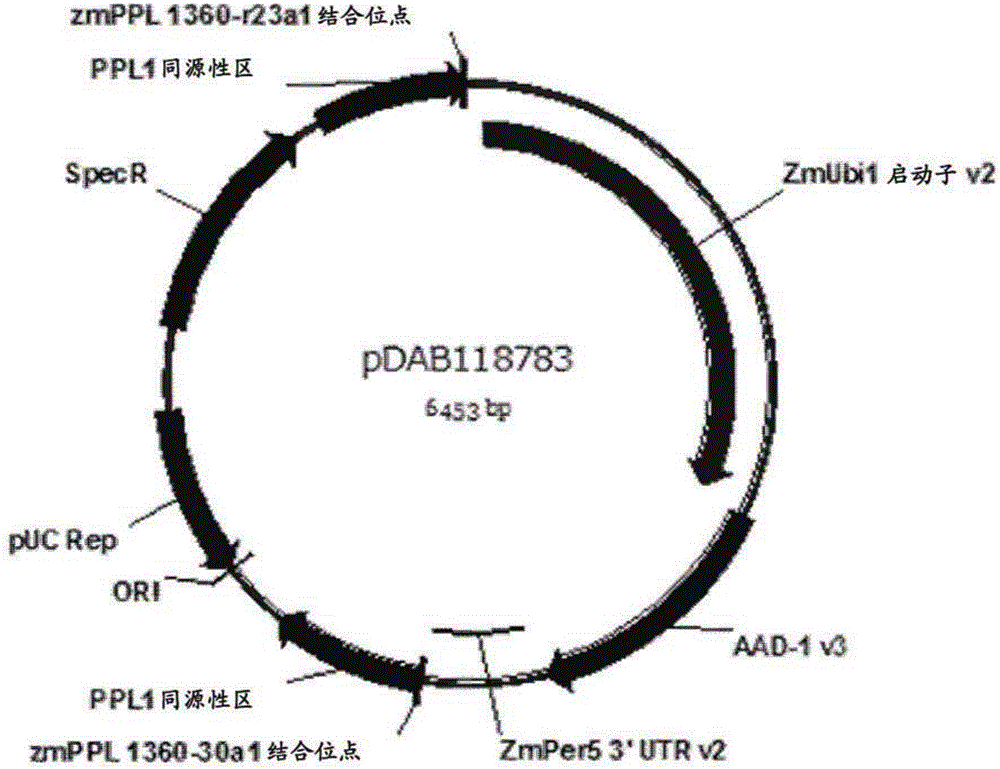
[0276] Example 3: Constructs for targeted genome modification in protoplasts isolated from haploid callus
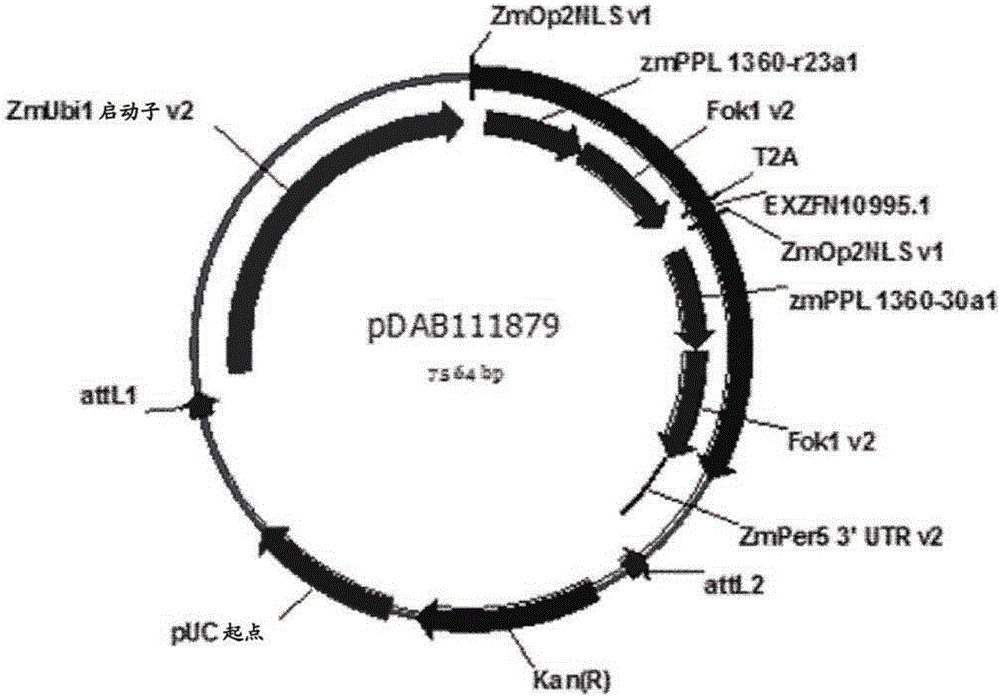
[0277] For targeted genome modification, the zinc finger nuclease (ZFN) construct pDAB111879 (depicted in figure 1 middle). Expression of the ZFN is driven by the maize ubi1 promoter and terminated with the maize per5 3'UTR. This expression cassette contains a "T2A" construct comprising two zinc finger monomer domains (zmPPL_1360-r23a1 and zmPPL_1360-30a1), encoded and expressed by a single coding region. The expressed transcript contains a T2A stutter signal (Mattion et al., 1996, J. Virol., 70:8124-7) to introduce a ribosomal stutter, which releases the first polypeptide during translation, and the designed To produce equimolar amounts of the first and second polypeptides upon further translation. The opaque2 nuclear localization sequence (NLS) was included in both ZF monomers for nuclear targeting. Both NLS-ZF domain fusions possess binding specificities for the u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com