Preparation and application of ultrasonically-controlled-release pluronic recombinant human tissue type plasiminogen activator
A technology of pluronic and plasminogen, applied in the field of medicine, can solve the problems of rebleeding, lack of treatment methods for hypertensive cerebral hemorrhage, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] The present invention provides a kind of preparation method of described sustained-release pharmaceutical preparation, comprises the following steps:
[0092] (i) providing a raw material component comprising:
[0093] (a) recombinant tissue plasminogen activator rt-PA;
[0094] (b) Pluronic F127; and
[0095] (c) water;
[0096] (ii) Mix the raw material components to form a sol / gel mixture, which is the sustained-release pharmaceutical preparation described in the first aspect of the present invention.
[0097] Wherein, the mixing described in step (ii) includes: mixing rt-PA with water to prepare a first solution containing rt-PA, and then mixing the first solution with Pluronic F127 to form a first mixture.
[0098] In addition, the method may also include the steps of:
[0099] (iii) Put the sustained-release pharmaceutical preparation prepared in the previous step into a medical container.
[0100] Application method
[0101] The invention is used to prepar...
Embodiment 1
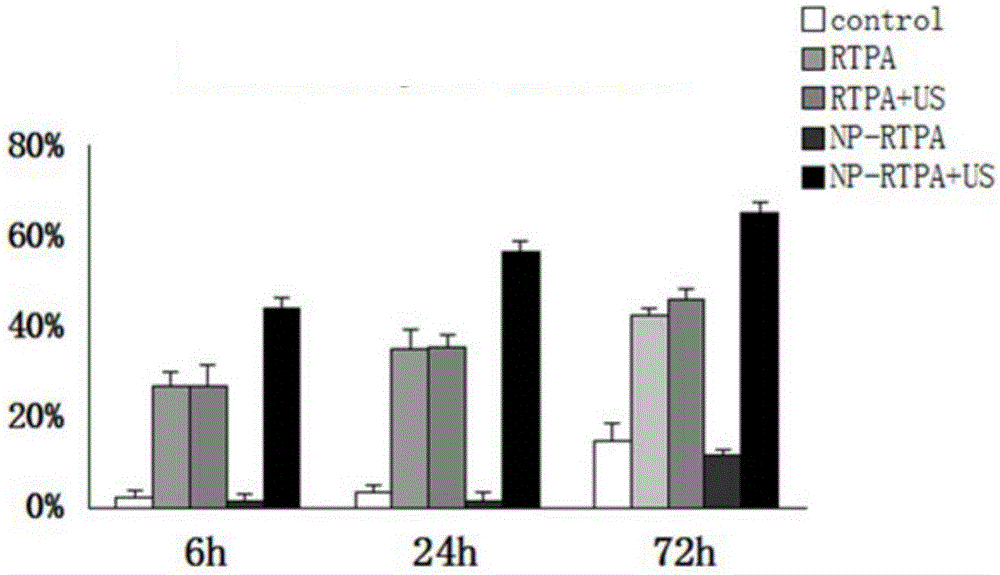
[0109] Example 1: Preparation of rt-PA-loaded nano-sustained-release pharmaceutical preparations and use of the preparations to complete in vitro hematoma dissolution
[0110] 1.1 Preparation of nano-sustained-release preparations loaded with rt-PA
[0111] At room temperature, commercially available rt-PA was dissolved with physiological saline to prepare a mixed solution with a concentration of 10 mg / ml. The Pluronic F-127 and the mixed solution are mixed according to the ratio of 190-290mg: 1ml (preferably, 230mg F-127 is mixed with 1ml of the mixed solution). Shake it on a vortex mixer for half an hour, and then place it in a refrigerator (5-15 degrees) for more than 8 hours to form a stable sol, store it in different containers according to the required dosage, and store it at room temperature or low temperature.
[0112] Experimental results: After Pluronic F127 is combined with rt-PA, the coating concentration ranges from 2-15mg / ml.
[0113] 1.2 Preparation of hematom...
Embodiment 2
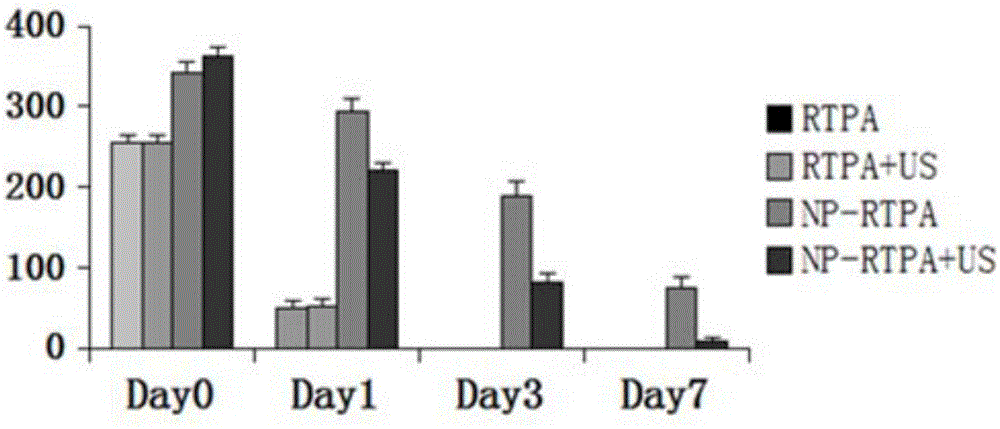
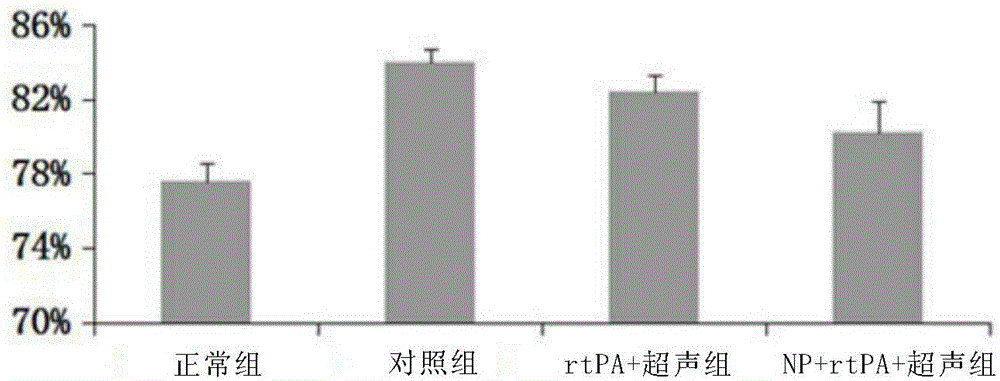
[0126] Example 2. Sustained-release administration of Pluronic F-127 loaded with rt-PA, completing the in vivo animal experiment of ultrasound-induced controlled release
[0127] 2.1 Research objects: Recombinant human tissue-type plasminogen activator (Recombinant human tissue-type plasminogen, rt-PA); Pluronic F-127 and 50 male SD rats.
[0128] 2.2 Research grouping: N=50, 10 male SD rats in each group.
[0129] (1) Sham operation group: intracerebral puncture but no hematoma injection;
[0130] (2) Simple hematoma group: inject 50 μl of autologous arterial blood into intracerebral hematoma;
[0131] (3) Hematoma + rt-PA group: 50 μl of autologous arterial blood + rt-PA were injected into the intracerebral hematoma;
[0132] (4) Hematoma + non-ultrasound group with rt-PA Pluronic F-127 system: Inject 50 μl of autologous arterial blood into the intracerebral hematoma + rt-PA Pluronic F-127 system;
[0133] (5) Hematoma + rt-PA Pluronic F-127 system ultrasound group: 50 μl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com