Vonoprazan fumarate single crystal, preparation method and uses thereof
A vonoprazan fumaric acid single crystal technology, applied in organic chemistry methods, digestive system, organic chemistry, etc., can solve the problems of high impurity content and difficult separation, and achieve good reproducibility, good shape, and guaranteed The effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of Crude Vonorazan Fumarate
[0030] According to the following two steps to synthesize the crude product of vonoprazan fumarate, the specific synthesis method is as follows:
[0031] Step 1 Synthesis of 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde, the specific method is as follows:
[0032] Add acetonitrile (50ml), 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (10g), 4-dimethylaminopyridine (1.3g) and N,N-diisopropyl to the reaction flask at room temperature Ethylamine (13 g), stirred at 40° C. to 50° C. for 2 hours, followed by TLC to complete the reaction. Add 1 mol / L hydrochloric acid solution to the reaction system to adjust the pH=4-5, then add water (60ml) and stir. Filtration and drying gave 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde (15.5 g, yellow solid).
[0033] Step 2 Synthesize the crude product of vonoprazan fumarate, the specific method is as follows:
[0034] Methanol (40ml)...
Embodiment 2
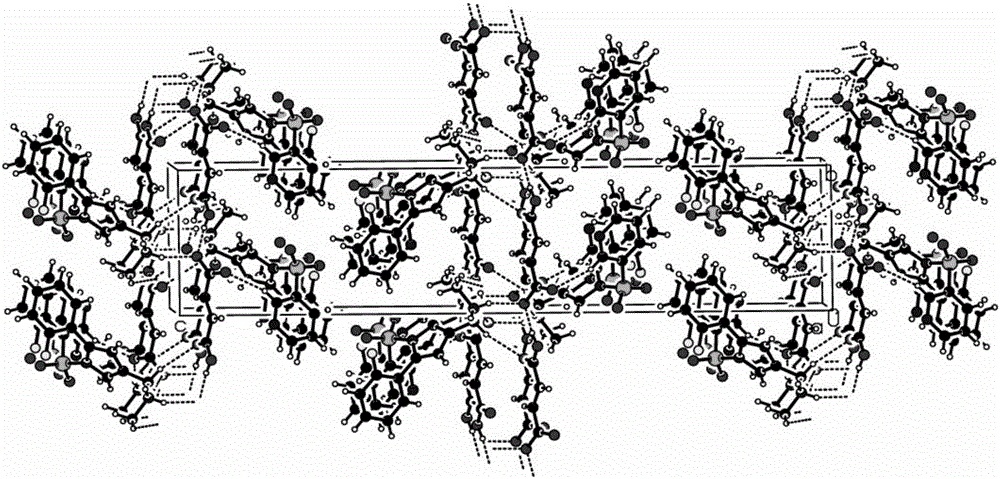
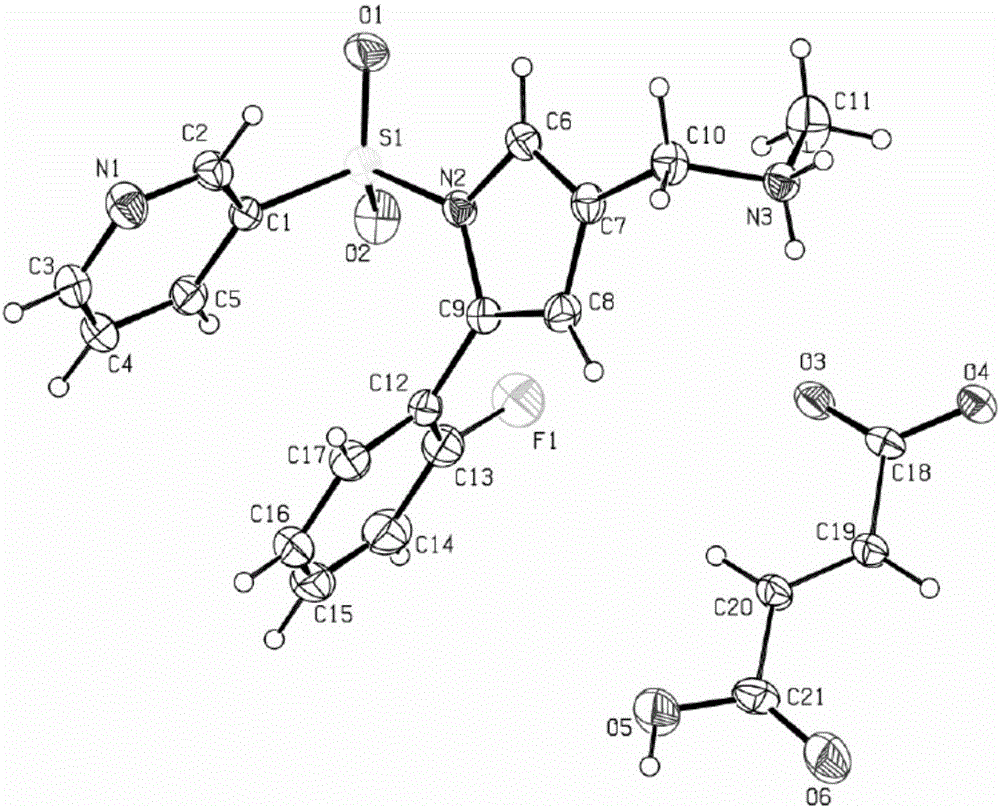
[0036] Example 2 Preparation of Vonoprazan Fumarate Single Crystal
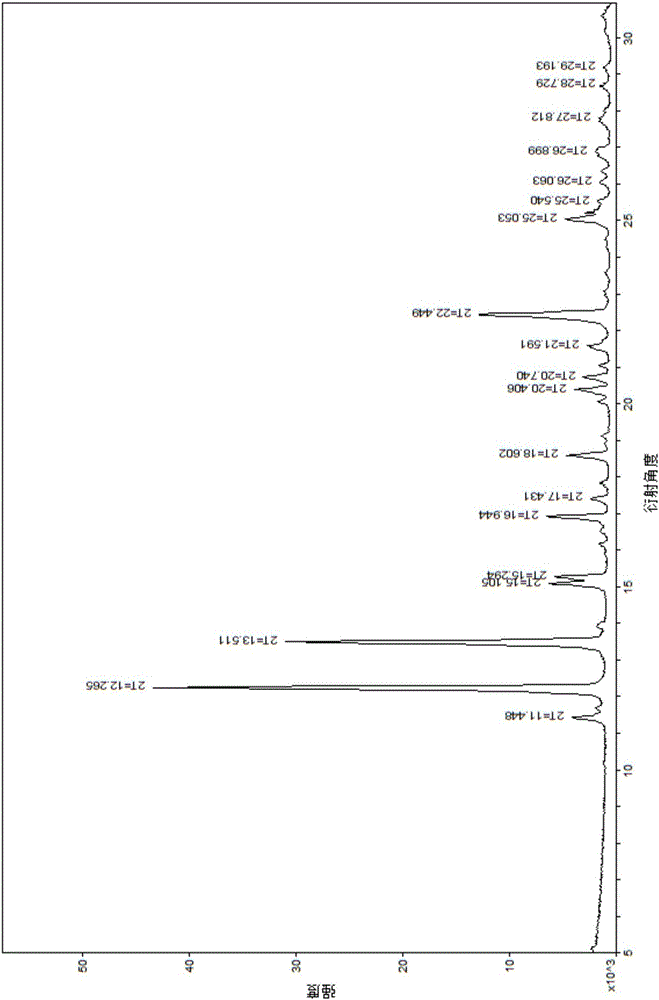
[0037] Weigh 1.0 g of the vonoprazan fumaric acid crude product obtained in Example 1, add the crystallization solvent methanol / water (5 ml / 5 ml) mixed solution, raise the temperature to 60 ° C ~ 70 ° C, and wait until the product is completely dissolved. In the evaporation method, the temperature was set at 25° C., and cultured for 5 days, colorless crystals were precipitated, and observed under a microscope, the crystals were vonoprazan fumaric acid single crystals, and the HPLC purity was 99.5%.
Embodiment 3
[0038] Example 3 Preparation of Vonoprazan Fumarate Single Crystal
[0039] Weigh 1.0 g of the vonoprazan fumaric acid crude product obtained in Example 1, add the crystallization solvent methanol / water (4 ml / 16 ml) mixed solution, raise the temperature to 60° C. to 70° C., and wait until the product is completely dissolved. In the evaporation method, the temperature was set at 30° C., and cultured for 10 days, a colorless crystal was precipitated, and observed under a microscope, the crystal was vonoprazan fumaric acid single crystal, and the HPLC purity was 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com