Di-oxime ester photoinitiator containing thiophene ring as well as preparation method and application thereof
A technology of photoinitiator and thiophene ring, which is applied in the field of photoinitiator, can solve the problems such as difficult curing of color resist, achieve the effects of improving sensitivity and thermal stability, good light absorption properties, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
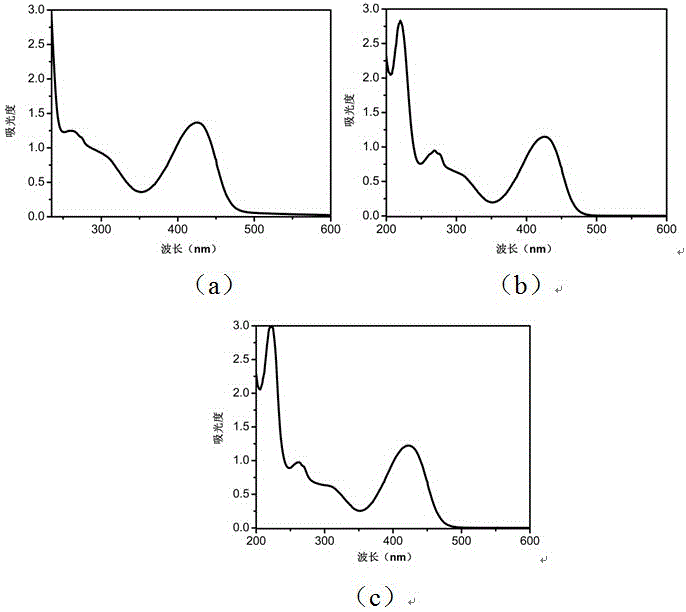
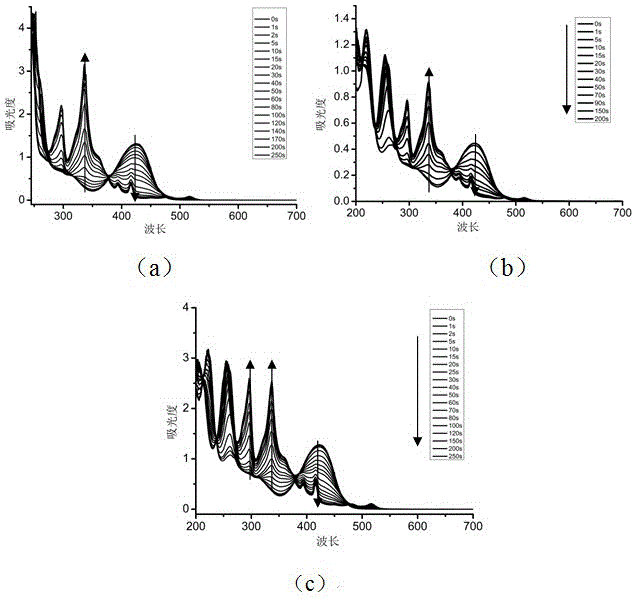
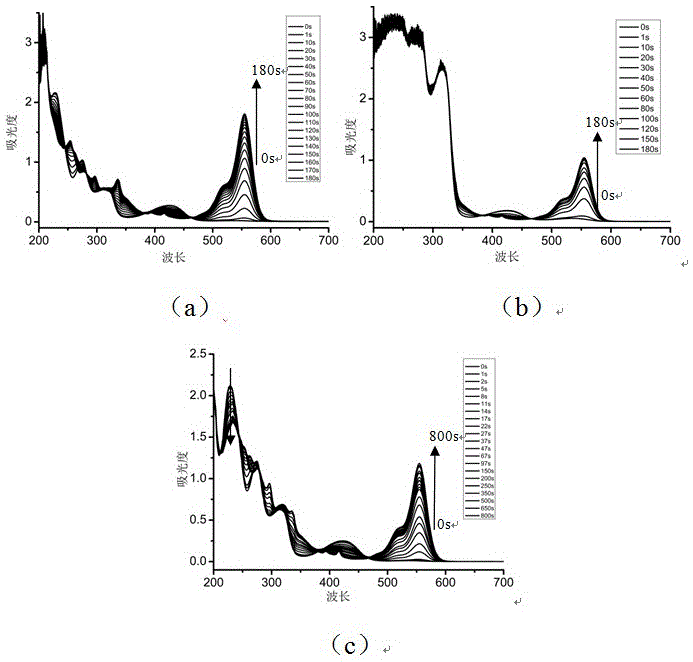
[0035] Preferred photoinitiator compounds of the present invention include the preparation of the following compound (1).
[0036] (1)
[0037] The initial substance is 1,3-benzenediacetonitrile, the intermediate oxime is obtained by reacting with and, and then the intermediate oxime is reacted with acid chloride by esterification to obtain the product. The used synthetic route of this preparation method is expressed as follows:
[0038]
[0039] a: Potassium hydroxide, methanol, tetrahydrofuran, room temperature, 24h;
[0040] b: Anhydrous tetrahydrofuran, anhydrous triethylamine, ice-water bath, dark, N 2 Protection, 2h.
[0041] (1) Synthesis of intermediate oxime: Dissolve nitrothiophene (3.23g, 0.02mol) in 120mL of methanol, add 6.8g of potassium hydroxide at room temperature, fully dissolve; Dissolve in 60ml of tetrahydrofuran, add dropwise to the methanol solution of nitrothiophene in a constant pressure dropping funnel, adjust the pH value to 5 with concentrat...
Embodiment 2
[0050] The preferred photoinitiator compounds of the present invention include the preparation of the following compound (2).
[0051] (2)
[0052] The initial substance is 1,3-benzenediacetonitrile, the intermediate oxime is obtained by reacting with and, and then the intermediate oxime is reacted with acid chloride by esterification to obtain the product. The used synthetic route of this preparation method is expressed as follows:
[0053]
[0054] a: Potassium hydroxide, methanol, tetrahydrofuran, room temperature, 24h;
[0055] b: Anhydrous tetrahydrofuran, anhydrous triethylamine, ice-water bath, dark, N 2 Protection, 2h.
[0056] (1) Step a is the same as (1) intermediate oxime synthesis in Preparation Example 1.
[0057] (2) Synthesis of the target molecule: under the protection of an inert gas, put 0.20 g (0.5 mmol) of the intermediate oxime obtained in step (1) into a dry flask, add 5 ml of anhydrous tetrahydrofuran, wrap it in aluminum foil to avoid light, a...
Embodiment 3
[0062] Preferred photoinitiator compounds of the present invention include the preparation of the following compound (3).
[0063] (3)
[0064] The preparation method was the same as in Example 2, except that in the process of preparing the photoinitiator, 2,4-difluorobenzenesulfonyl chloride was used instead of p-toluyl chloride in the second step, and the other preparation methods remained unchanged, with a yield of 40%. The specific NMR characterization results are as follows:
[0065] 1 H NMR (400 MHz, CDCl 3 ) δ: 8.12 (ddd, J = 14.0, 8.7, 5.8 Hz, 2H), 7.52 –7.38 (m, 4H), 7.05 – 6.97 (m, 2H), 6.97 – 6.88 (m, 4H), 6.47 (t, J = 4.2 Hz, 2H);
[0066] 13 C NMR (101 MHz, CDCl 3 ) δ: 164.93, 163.71, 160.64, 143.15, 141.28, 134.25, 131.33, 128.54, 126.13, 125.66, 124.62, 122.81, 118.81, 112.43, 106.57, 106.01.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com