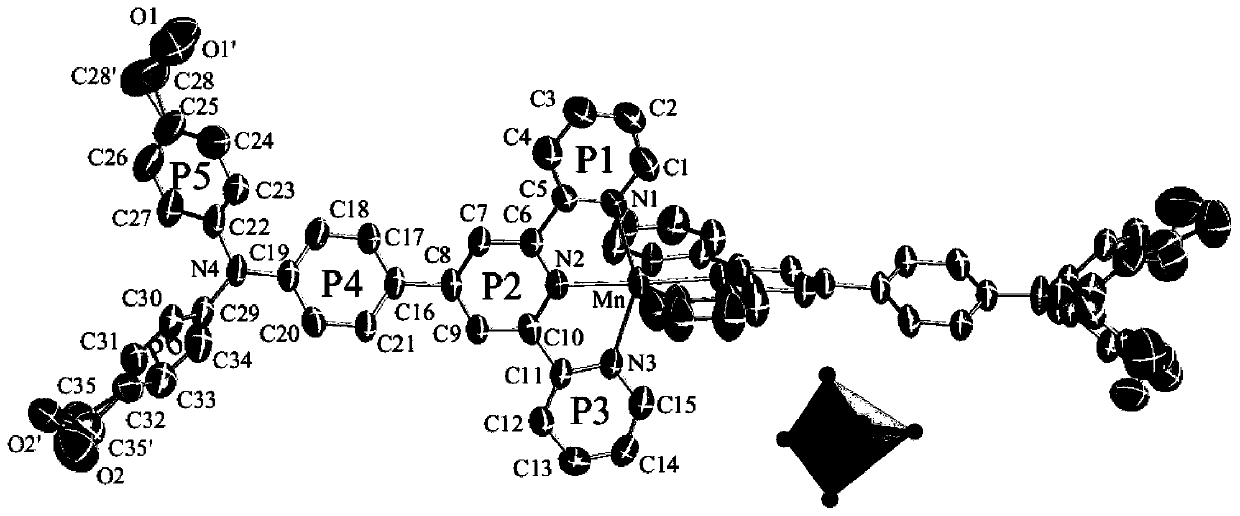
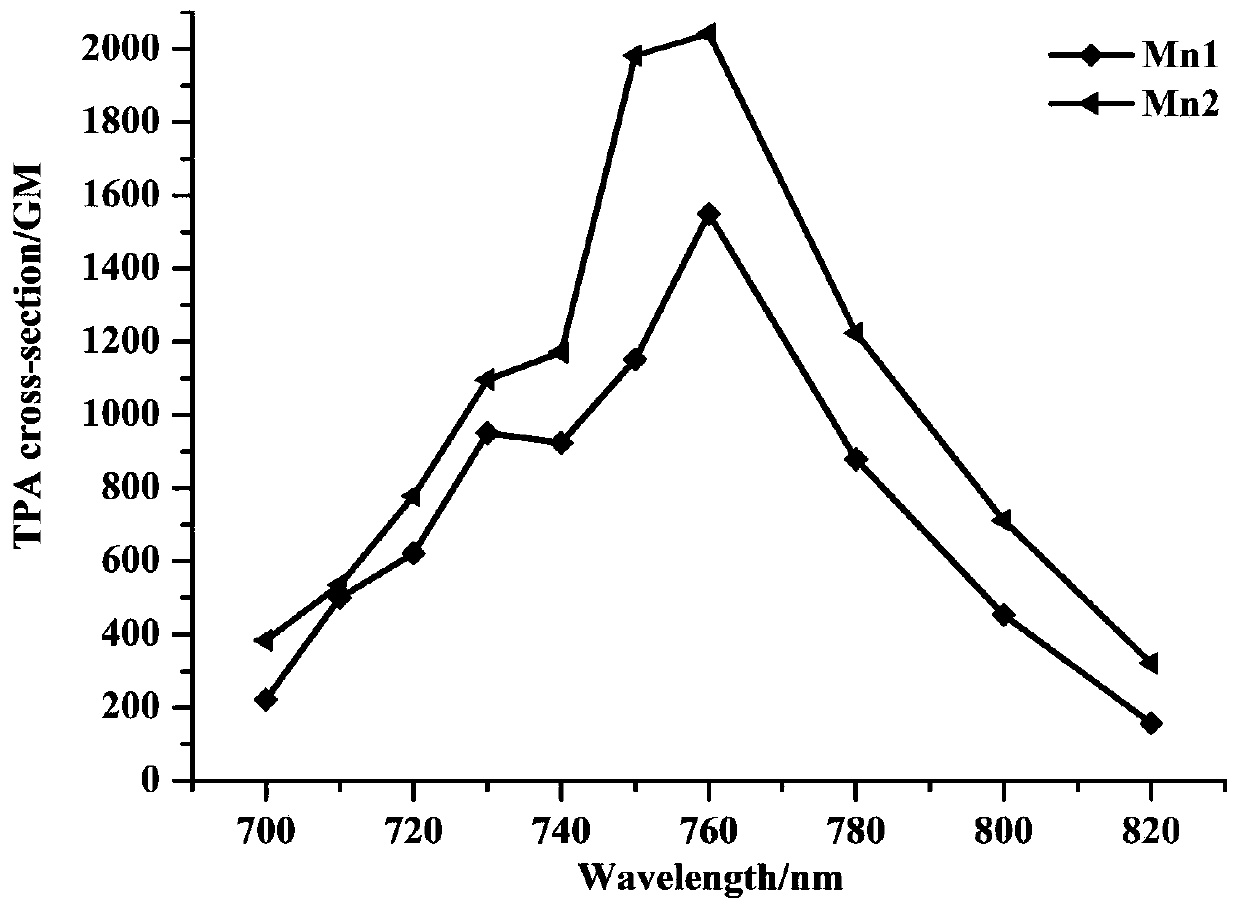
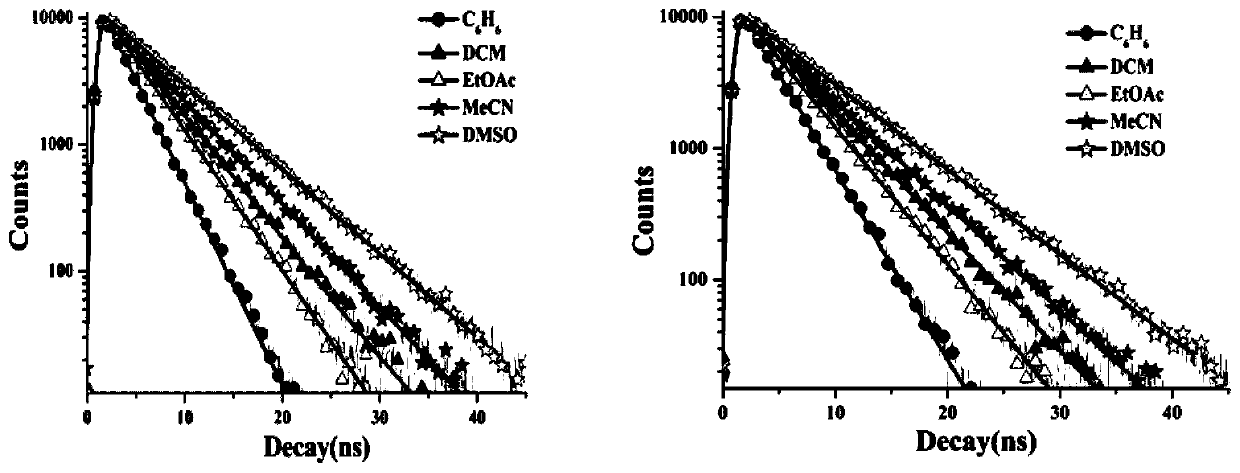
A triphenylamine terpyridine manganese complex with dual functions of two-photon imaging and magnetic resonance imaging and its synthesis method
A terpyridine manganese and magnetic resonance technology, which is applied in organic chemistry, particle and sedimentation analysis, and material analysis, can solve the problems of low sensitivity and achieve the effects of low excitation energy, long fluorescence lifetime, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Ligand L 1 (0.202g, 0.400mmol) was dissolved in 20mL of absolute ethanol and placed in a 50mL round-bottomed flask, added with Mn(OAc) 2 4H 2 O (0.049g, 0.200mmol) in 20mL ethanol solution, heated to reflux for 4h, after the reaction, the reaction solution was cooled to room temperature, and then NH 4 PF 6 (0.082g, 0.500mmol), continue heating to reflux reaction for 2h, precipitate a red solid, filter while it is hot, collect the filter cake by filtration under reduced pressure, and recrystallize with absolute ethanol to obtain the target product Mn10.206g as a red solid, the yield 75.90% (calculated based on the amount of ligand, the same below).
[0045] M.P.: 232℃. 1 H NMR (400MHz, d 6 -DMSO,ppm):δ8.72(d,J=31.1Hz,12H),8.04(s,4H),7.86(s,4H),7.52(s,4H),7.34(s,8H),7.11( s,14H),5.15(s,2H),4.49(s,4H).IR(cm -1):3421(m),3063(m),2922(m),1587(vs),1512(s),1475(s),1417(m),1329(s),1288(m),1196(s ),1017(m),844(s),792(s),760(m),698(m),658(m),640(m),558(s),522(m).ESI-MS :m...
Embodiment 2
[0047] Ligand L 2 (0.214g, 0.400mmol) was dissolved in 20mL of absolute ethanol and placed in a 50mL round-bottomed flask, added with Mn(OAc) 2 4H 2 O (0.049g, 0.200mmol) in 20mL ethanol solution, heated to reflux for 4h, after the reaction, the reaction solution was cooled to room temperature, and then NH 4 PF 6 (0.082g, 0.500mmol), continue heating to reflux reaction for 2h, precipitate a red solid, filter while hot, collect the filter cake by filtration under reduced pressure, and recrystallize with absolute ethanol to obtain the target product Mn20.211g, which is a red solid, yield 74.45% (calculated based on the amount of ligand, the same below).
[0048] M.P.: 236℃. 1 H NMR (400MHz, d 6 -DMSO,ppm):δ8.72(d,J=30.9Hz,12H),8.03(s,4H),7.85(s,4H),7.52(s,4H),7.32(s,8H),7.09( s,13H),5.15(s,4H),4.49(s,8H).IR(cm -1 ):3393(m),3063(m),2922(m),2872(m),1593(vs),1510(s),1475(s),1417(m),1327(s),1288(m ),1197(s),1016(s),844(vs),791(s),731(m),671(m),658(m),639(m),558(s),521(m ).E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com