A membrane-permeable dye with a large two-photon fluorescence active cross-section and its application
A two-photon fluorescence and permeability technology, applied in the direction of luminescent materials, fluorescence/phosphorescence, styrene-based dyes, etc., can solve the problems that restrict two-photon three-dimensional imaging technology for cell biological imaging and observation, and achieve excellent cell membrane permeability , excellent low toxicity, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of 4,4-2 Formyl Triphenylamine
[0028] 20ml of DMF was added to the single neck flask. Under stirring in an ice-water bath, 20 ml of phosphorus oxychloride was slowly added dropwise to the above solution, and stirred at room temperature for 1 h to obtain a yellow turbid liquid. 5 g (20.4 mmol) of triphenylamine was weighed, dissolved in 10 ml of chloroform, and then gradually added to the above-mentioned stirred solution, and the reaction was heated under reflux for 10 hours. The reaction solution was poured into 1000 mL of water, and CH 2 Cl 2 extraction. The organic layer was washed with saturated sodium chloride solution, anhydrous MgSO 4 Dry, filter and evaporate the solvent. The crude product was separated by column chromatography, and petroleum ether / ethyl acetate (10:1) was used as the eluent to obtain a light yellow solid, which was 4,4-2 formyl triphenylamine. Yield: 55%.
[0029] 1 H NMR (400MHz, d6-DMSO): δ (ppm) 9.88 (s, 2H), 7.85 (d, J=8....
Embodiment 2
[0037] 4-[(E)-2-(1H-benzimidazol-2-yl)vinyl]-N-{4-[(E)-2-(1H-benzimidazol-2-yl)vinyl] Synthesis of Phenyl}-N-phenylaniline
[0038] The synthesis technique is the same as in Example 1, and 4-[(E)-2-(1H-benzimidazol-2-yl)vinyl]-N-{4-[(E)-2-(1H-benzoyl) is obtained by synthesis Imidazol-2-yl)vinyl]phenyl}-N-phenylaniline, yield: 17%.
[0039] 1H NMR (400MHz, d6-DMSO): δ (ppm): 12.55 (s, 2H), 7.65 (m, J=7.67Hz, 6H), 7.55 (d, J=7.64Hz, 4H), 7.41 (t, J=7.84Hz, 2H), 7.16 (t, J=7.76Hz, 5H), 7.11 (d, J=7.68Hz, 4H), 7.07 (d, J=8.24Hz, 4H).
Embodiment 3
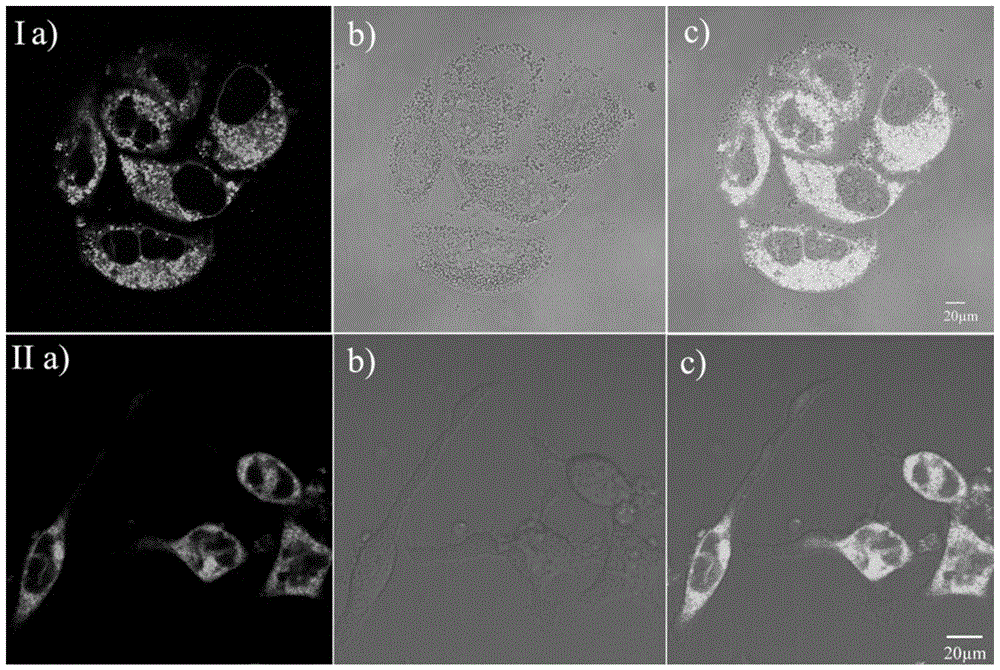
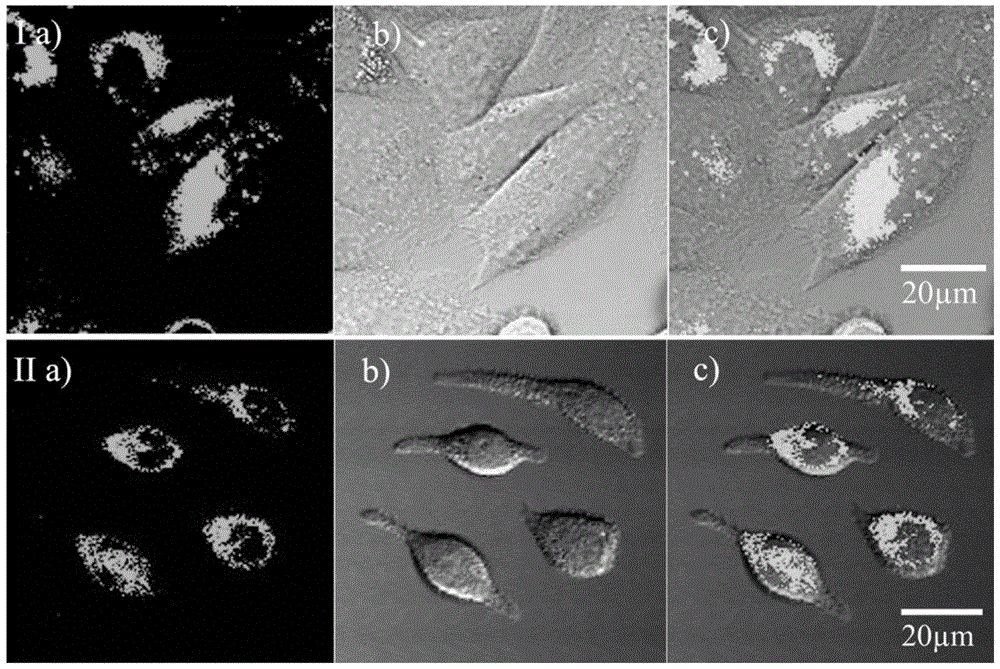
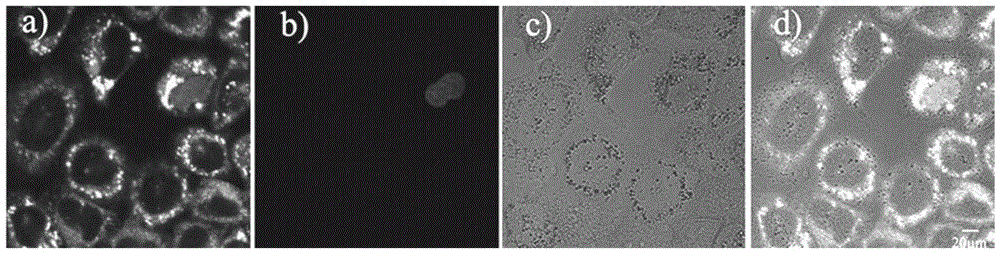
[0041] RBL-2H3 and Hela cell culture
[0042] RBL-2H3 or HeLa cell lines were adherently cultured in medium containing 10% fetal bovine serum at 37°C, 5% CO 2 Cultured in a saturated humidity incubator, and the medium was changed every 2 to 3 days for passage. After the cells grow to the logarithmic phase, the slicing culture: ① Soak the coverslip in absolute ethanol for 30 minutes, dry it in an alcohol lamp and put it into a disposable 35mm petri dish; ② Wash the cells in the 100ml cell flask with PBS Three times, digest with 1 ml of 0.25% trypsin for 3-5 minutes, carefully pour out the medium, add a small amount of fresh medium and pipet evenly, after counting the cells, leave the cells of the appropriate density, and add the medium to the desired volume ( Control cells at a final concentration of 1x10 5 ), inoculated into a Petri dish containing a coverslip, placed in CO 2 Cultured in an incubator to allow the cells to grow on the sheet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com