Membrane permeability dye with large two-photon fluorescence active cross section and application of membrane permeability dye
A two-photon fluorescence and permeability technology, applied in the direction of luminescent materials, fluorescence/phosphorescence, styrene-based dyes, etc., can solve the problems that restrict two-photon three-dimensional imaging technology for cell biological imaging and observation, and achieve excellent cell membrane permeability , low excitation energy, and large two-photon fluorescence active absorption cross-section
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of 4,4-2 formyl triphenylamine
[0028] 20ml of DMF was added to the one-necked flask. Under stirring in an ice-water bath, 20 ml of phosphorus oxychloride was slowly added dropwise to the above solution, and stirred at room temperature for 1 h to obtain a yellow turbid solution. Weigh 5 g (20.4 mmol) of triphenylamine, dissolve it with 10 ml of chloroform, and then gradually add it into the above stirred solution, and heat to reflux for 10 hours. The reaction solution was poured into 1000 mL of water, and the 2 Cl 2 extraction. The organic layer was washed with saturated NaCl solution, anhydrous MgSO 4 Dry, filter and evaporate the solvent. The crude product was separated by column chromatography with petroleum ether / ethyl acetate (10:1) as the eluent to obtain a light yellow solid, namely 4,4-2 formyltriphenylamine. Yield: 55%.
[0029] 1 HNMR (400MHz, d6-DMSO): δ (ppm) 9.88 (s, 2H), 7.85 (d, J = 8.64Hz, 4H), 7.49 (t, J = 7.84Hz, 2H), 7.33 (t, J = 7...
Embodiment 2
[0037] 4-[(E)-2-(1H-benzimidazol-2-yl)vinyl]-N-{4-[(E)-2-(1H-benzimidazol-2-yl)ethenyl] Synthesis of phenyl}-N-phenylaniline
[0038] The synthesis process is the same as in Example 1, and 4-[(E)-2-(1H-benzimidazol-2-yl)vinyl]-N-{4-[(E)-2-(1H-benzo Imidazol-2-yl)vinyl]phenyl}-N-phenylaniline, yield: 17%.
[0039] 1H NMR (400MHz, d6-DMSO): δ (ppm): 12.55 (s, 2H), 7.65 (m, J = 7.67Hz, 6H), 7.55 (d, J = 7.64Hz, 4H), 7.41 (t, J =7.84Hz, 2H), 7.16(t, J=7.76Hz, 5H), 7.11(d, J=7.68Hz, 4H), 7.07(d, J=8.24Hz, 4H).
Embodiment 3
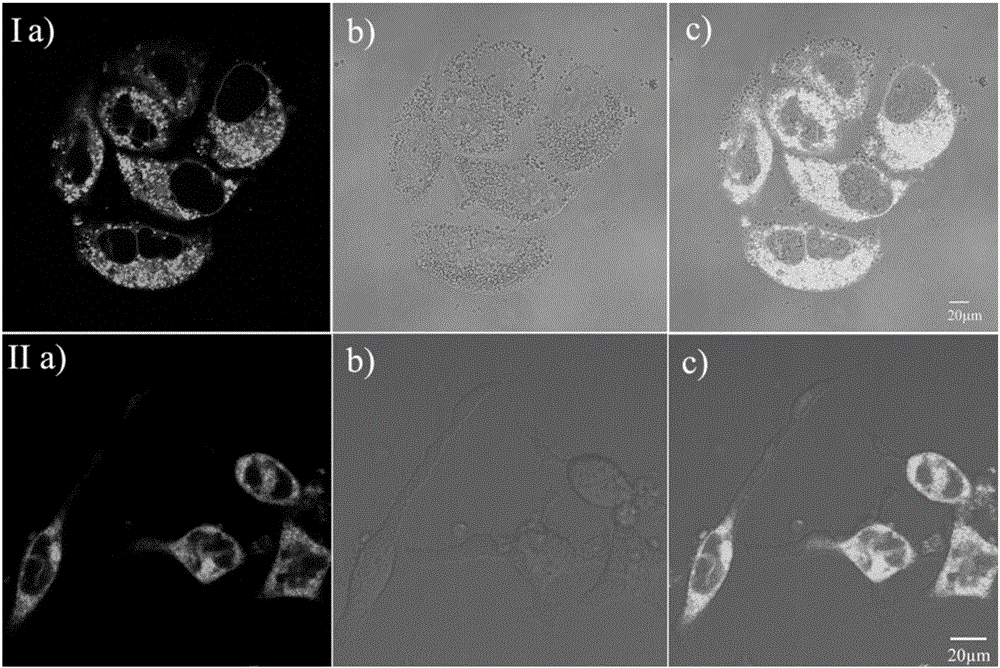
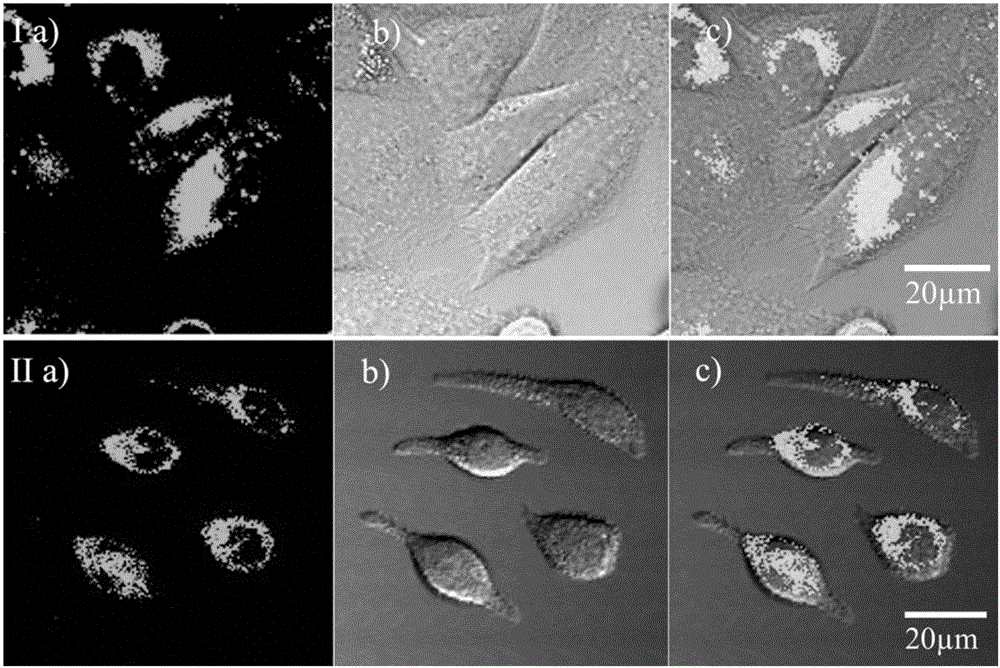
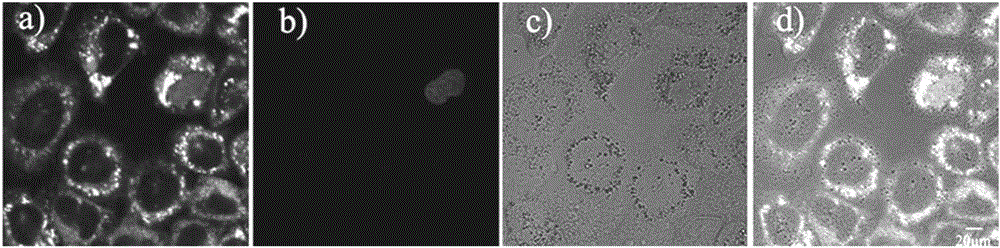
[0041] RBL-2H3 and Hela cell culture
[0042] Adherent culture of RBL-2H3 or HeLa cell line in 10% fetal bovine serum medium, at 37°C, 5% CO 2 Cultured in a saturated humidity incubator, and subcultured once every 2-3 days. After the cells grow to the logarithmic phase, the splicing culture: ① Soak the coverslips in absolute ethanol for 30 minutes, dry them with an alcohol lamp, and put them into a disposable 35mm culture dish; ② Wash the cells in the 100ml cell bottle with PBS. Three times, digest with 1ml 0.25% trypsin for 3-5 minutes, pour out the medium carefully, add a small amount of fresh medium and pipette evenly, after counting the cells, leave cells with a suitable density, and add the medium to the required volume (The final concentration of control cells was 1x10 5 ), inoculated into a petri dish containing a coverslip, and placed in CO 2 Cultured in an incubator to allow the cells to grow on the sheet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com