Synthesis method for preparing PARP inhibitor Niraparib
A synthesis method and an inhibitor technology, which are applied in the field of synthesis of the PARP inhibitor Niraparib, can solve problems such as difficulty in realizing large-scale industrial production, restrictions on industrial safety production, and long synthesis routes, and achieve easy large-scale production and industrialization The effect of large production and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
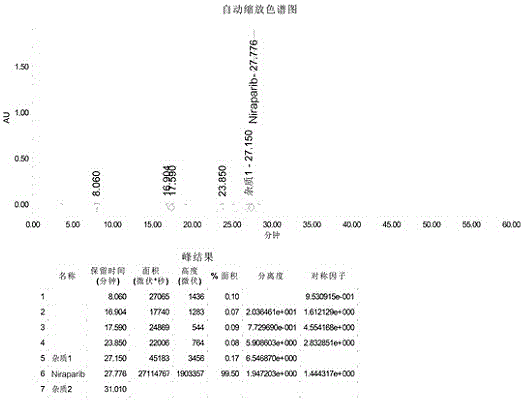
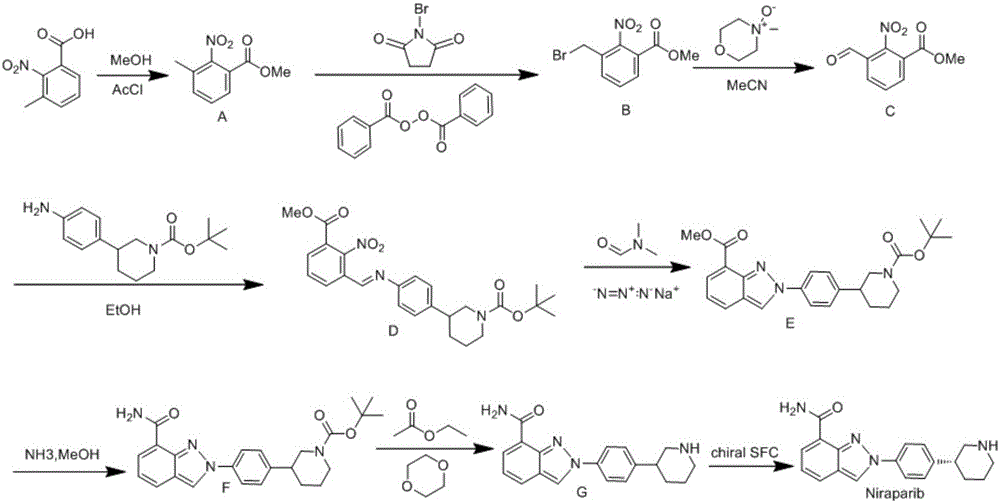
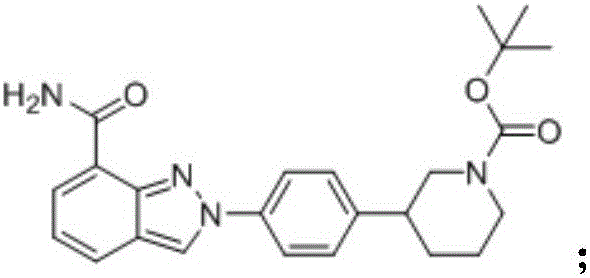
[0034] Intermediate (II) The synthesis of intermediate (II) is divided into two steps, the preparation of the first step diazonium salt: 52mL (0.4mol) methyl anthranilate is mixed with 170mL 20% sulfuric acid (0.62mol), cooled to At 10°C, slowly add 42 mL of 30% sodium nitrite (0.4 mol) solution dropwise into the reaction flask. Check the excess nitrous acid with starch potassium iodide test paper to determine the end point of the reaction. The generated brown-red diazonium salt carries out the coupling reaction of the second step with tert-butyl-3-phenylpiperidine-1-carboxylate: get 100g (0.4mol) tert-butyl-3-phenylpiperidine- 1-tert-butyl formate was dissolved in 500mL of 10% sodium hydroxide solution, and was dripped into the above-mentioned diazonium salt under constant stirring, stirred for 5h, left to stand for crystallization, suction filtered, and recrystallized in ethanol to obtain 127g of the intermediate (II ), yield: 78%.
[0035] 1 H-NMR (400MHz, DMSO-d6) (pp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com