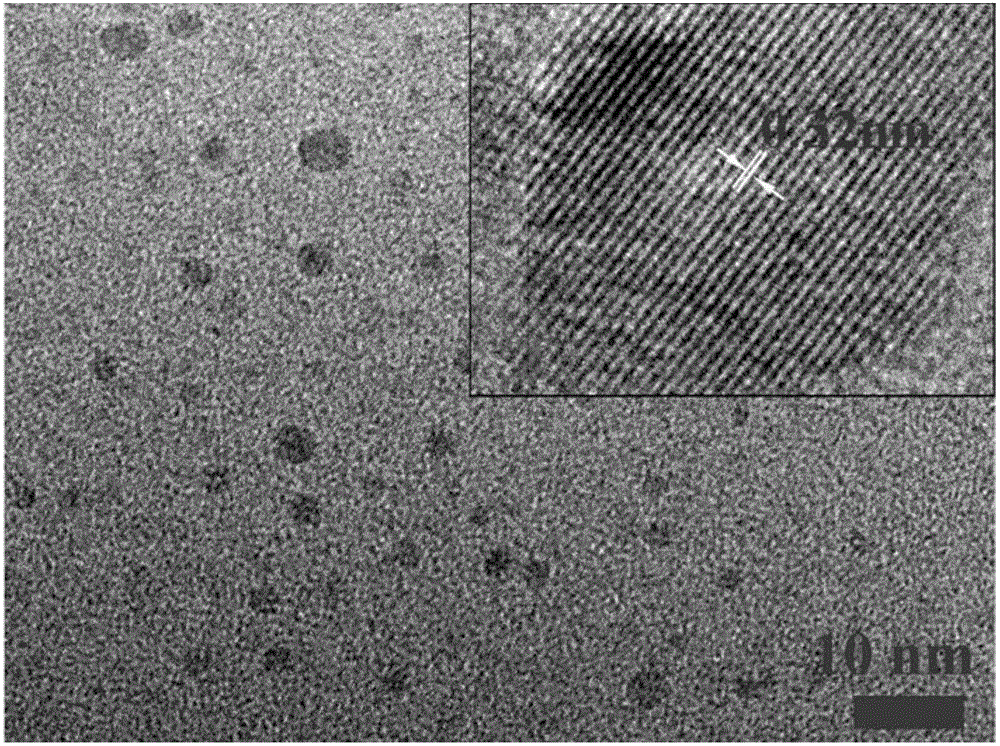
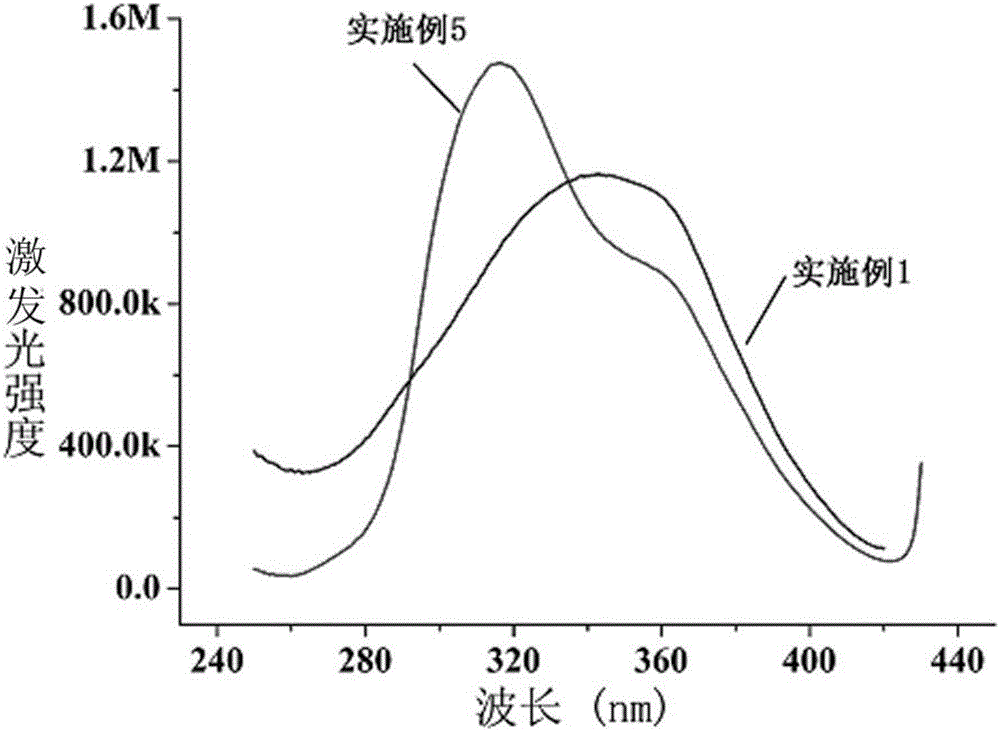
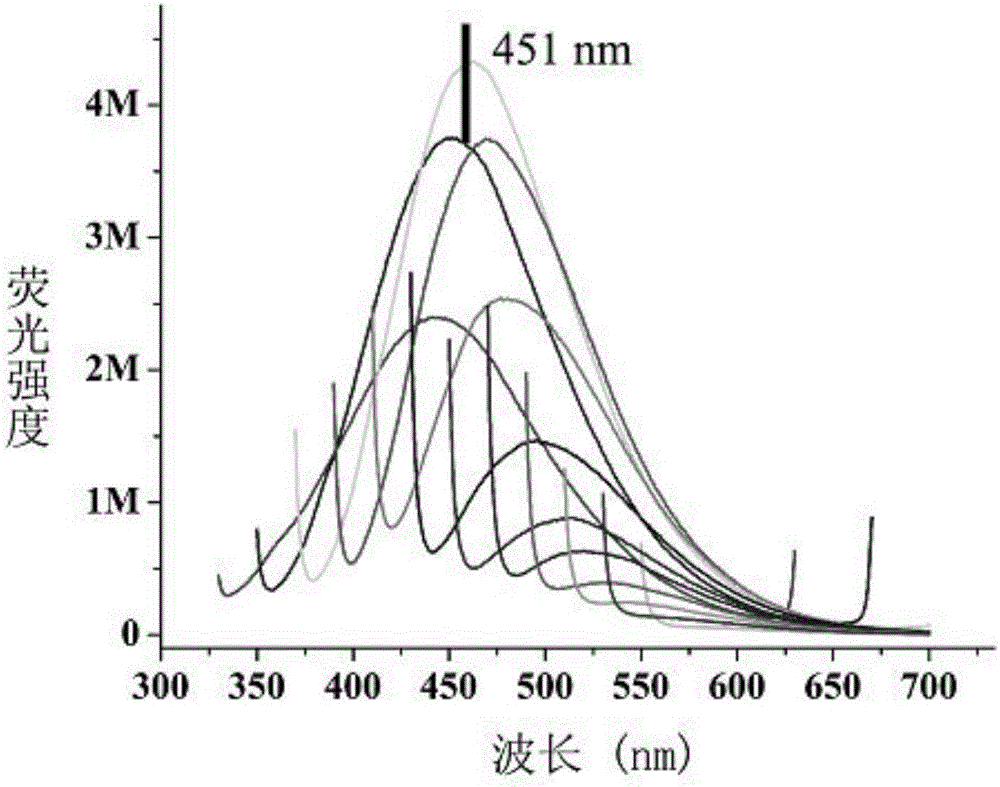
A carbon quantum dot preparing method
A technology of carbon quantum dots and dry matter, which is applied in the field of preparation of carbon quantum dots, can solve the problems of low yield of carbon quantum dots, many impurities of carbon quantum dots, and is not suitable for wide application, etc., to achieve excellent chemical stability, reduce Production cost, the effect of easy promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for preparing carbon quantum dots, comprising the following steps:
[0033] Pretreatment of raw materials: Clean the waste paper in an ultrasonic cleaning machine. The solvent for the first cleaning is ethanol, and the cleaning time is 5 minutes. The solvent for the second cleaning is deionized water, and the cleaning time is 20 minutes. Dried under hot air to obtain a dried product with a moisture content of 3%;
[0034] Hydrothermal reaction: put 1g of dry matter in an autoclave, add 45mL of 2.3mol / L NaOH solution, seal it, then put the autoclave in an oven, and conduct a hydrothermal reaction at 210°C for 3h;
[0035] Filtration: After the autoclave is cooled, take it out, and filter the mixed solution in the autoclave with double-layer filter paper to obtain the filtrate;
[0036] Centrifugation: centrifuge the filtrate at a speed of 14500r / min for solid-liquid separation for 15min, filter to obtain the supernatant;
[0037] Dialysis: Put the supernatant ...
Embodiment 2
[0041] A method for preparing carbon quantum dots, comprising the following steps:
[0042] Pretreatment of raw materials: Clean the waste paper in an ultrasonic cleaning machine. The solvent for the first cleaning is ethanol, and the cleaning time is 10 minutes. The solvent for the second cleaning is deionized water, and the cleaning time is 25 minutes. Dried under hot air to obtain a dried product with a moisture content of 5%;
[0043] Hydrothermal reaction: put 1.2g of dry matter in an autoclave, add 50mL of 2.5mol / L NaOH solution, seal it, then put the autoclave in an oven, and conduct a hydrothermal reaction at 220°C for 3.5h;
[0044] Filtration: After the autoclave is cooled, take it out, and filter the mixed solution in the autoclave with double-layer filter paper to obtain the filtrate;
[0045] Centrifugation: centrifuge the filtrate at a speed of 15000r / min for solid-liquid separation for 20min, filter to obtain the supernatant;
[0046] Dialysis: Put the superna...
Embodiment 3
[0049] A method for preparing carbon quantum dots, comprising the following steps:
[0050] Pretreatment of raw materials: Clean the waste paper in an ultrasonic cleaning machine. The solvent for the first cleaning is ethanol, and the cleaning time is 8 minutes. The solvent for the second cleaning is deionized water, and the cleaning time is 23 minutes. Under hot air drying, the dried product with a moisture content of 4% is obtained;
[0051] Hydrothermal reaction: put 1.1g of dry matter in an autoclave, add 48mL of 2.4mol / L NaOH solution, seal it, then put the autoclave in an oven, and conduct a hydrothermal reaction at 215°C for 3.25h;
[0052] Filtration: After the autoclave is cooled, take it out, and filter the mixed solution in the autoclave with double-layer filter paper to obtain the filtrate;
[0053] Centrifugation: centrifuge the filtrate at a speed of 14500r / min for solid-liquid separation for 18min, filter to obtain the supernatant;
[0054] Dialysis: Put the sup...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com