Rate type difunctional fluorescence molecular probe for detecting HSO4- ions, SO2 and derivatives thereof
A fluorescent molecular probe, SO2 technology, applied in the field of analytical chemistry, can solve the problems of difficult separation and purification, slow development of multifunctional probes, and high preparation costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis of 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indol-3-ium iodide.
[0031] Add 10 g (47.78 mmol) of 1,1,2-trimethyl-1H-benzo[e]indole and 8.94 g (57.34 mmol) of ethyl iodide in a 100 mL round bottom flask, 40 mL of acetonitrile, and load Reflux the condenser, heat and stir to reflux for 20 hours; cool the reaction solution to room temperature, filter, wash the filter cake with ether, and dry in vacuo to obtain 3-ethyl-1,1,2-trimethyl-1H-benzo[e ] Indol-3-ium iodide 14.6 g (83.7% yield).
Embodiment 2
[0032] Example 2 Synthesis of 3-ethyl-2-(4-hydroxystyryl)-1,1-dimethyl-1H-benzo[e]indol-3-ium iodide
[0033] Into a 25 mL round bottom flask was added 3.0 g (8.21 mmol) 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indol-3-ium iodide and 1.2 g (9.86 mmol) Dissolve p-hydroxybenzaldehyde in 15 mL of absolute ethanol, add 3-4 drops of piperidine or pyrrolidine, and stir at room temperature for 12 hours; filter the reaction solution, wash with ethanol, and dry in vacuum to obtain the fluorescent molecular probe compound 2.98 of the present invention. g (77.2% yield). 1 H NMR (500 MHz, DMSO- d 6 ) δ 8.55 - 8.43 (d, J = 16.1Hz, 1H), 8.43 - 8.37 (d, J = 8.5 Hz, 1H), 8.31 - 8.25 (d, J = 9.0 Hz, 1H),8.24 - 8.18 (d, J = 8.2 Hz, 1H), 8.18 - 8.12 (d, J = 8.8 Hz, 2H), 8.11 - 8.03(d, J = 8.9 Hz, 1H), 7.83 – 7.76 (ddd, J = 8.4, 6.9, 1.3 Hz, 1H), 7.73 - 7.66(t, J = 7.5 Hz, 1H), 7.54 - 7.40 (d, J = 16.1 Hz, 1H), 7.00 - 6.89 (d, J =8.4 Hz, 2H), 5.10 - 4.48 (q, J = 7.2 Hz, 2H), 2.2...
Embodiment 3
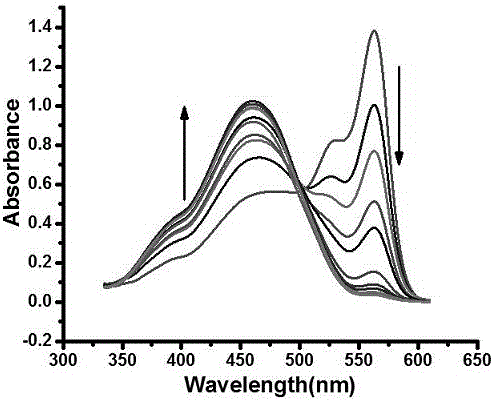
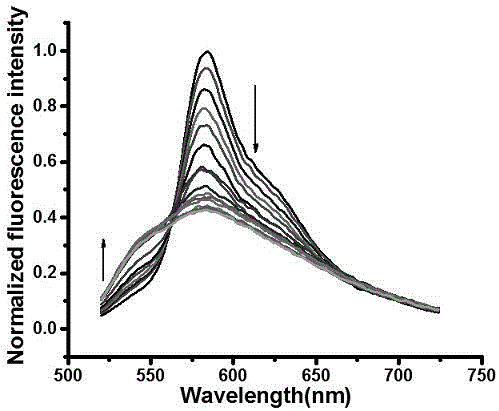
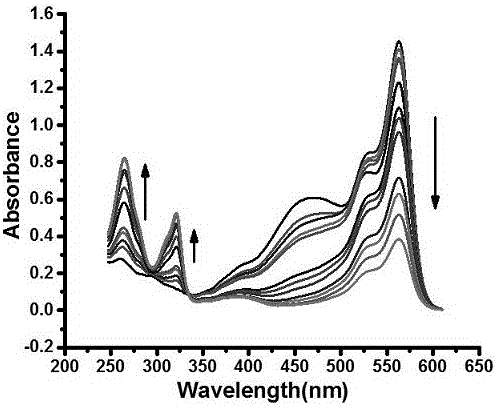
[0034] Example 3 Dual-functional probes in the detection of HSO 4 - Applications in ions.
[0035] Spectral property experiment of fluorescent molecular probes according to the present invention: dissolving the probes in dimethyl sulfoxide (DMSO) to configure 1 mM probe solutions, and configuring 1 mM NaHSO respectively 4 aqueous solution and Na 2 SO 3 Aqueous solution, and 10 mM interfering ion aqueous solution, all tests are tested at room temperature with a volume ratio of organic phase to aqueous phase of 8:2, the organic phase is dimethyl sulfoxide (DMSO) with 1 mM probe solution and detection The ethanol added at the time, the water phase is HSO 4 - and SO 2 Aqueous solution and deionized water added during detection. The specific test method is: take 20 μL of 1 mM probe solution, 1580 μL of analytical pure ethanol, 20 μL of 1mM NaHSO 4 Water solution and 380 μL deionized water solution were placed in a 2 mL sample tube, and the changes in UV absorption and fluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com