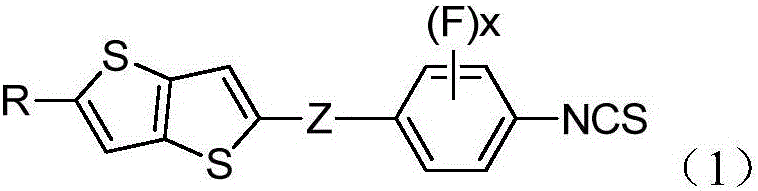
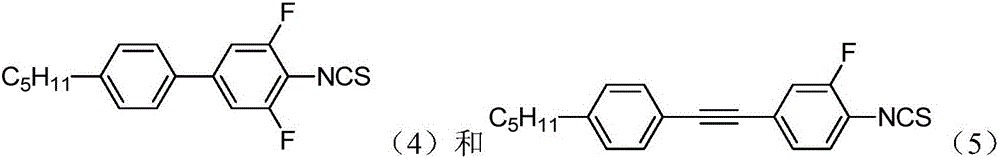Thiophthene high birefringence liquid crystal compound and composition thereof
A technology of liquid crystal compounds and liquid crystal compositions, which is applied in the direction of liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as difficult to meet application requirements and low birefringence, and achieve increased optical anisotropy and high birefringence The effect of high rate and wide liquid crystal phase range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Synthesis of 2-(3,5-difluoro-4-isothiocyanatophenyl)-5-pentylthieno[3,2-b]thiophene:
[0057] The specific structural formula is as follows:
[0058]
[0059] The preparation process is as follows:
[0060] (1) Step 1: Synthesis of 1-(thieno[3,2-b]thiophen-2-yl)pentyl-1-one
[0061]
[0062] Add thieno[3,2-b]thiophene (100.0 g, 0.713 mol) and 1 L of dichloromethane into a 2 L three-necked flask equipped with mechanical stirring and a constant pressure dropping funnel, stir and dissolve thoroughly, and then start to drop at room temperature Add n-pentanoyl chloride (130.6g, 1.084mol), add anhydrous aluminum trichloride (142.6g, 1.70mol) in batches after dripping, after continuing to react for 2h, TLC monitors the reaction, after the reaction finishes, quench with ice-water mixture The reaction was quenched, extracted with dichloromethane, washed with saturated sodium bicarbonate solution, washed with water until neutral, dried over anhydrous magnesium ...
Embodiment 2
[0078] Example 2: Synthesis of 2-(3-fluoro-4-isothiocyanatophenyl)-5-pentylthieno[3,2-b]thiophene:
[0079]
[0080] Using 2-fluoro-4-iodoaniline to replace 2,6-difluoro-4-iodoaniline in step (4) of Example 1, the same method is used to synthesize 2-(3-fluoro-4-isothiocyanatobenzene base)-5-pentylthieno[3,2-b]thiophene.
[0081] Structure Identification: 1 H NMR (δ, CDCl 3 ): 7.411(s, 1H), 7.355~7.381(dd, 1H, J 1 = 2Hz,J 2 = 11Hz), 7.317 ~ 7.338 (dd, 1H, J 1 = 2Hz,J 2=8.5Hz), 7.163~7.195(t, 1H, J=8Hz), 6.945(s, 1H), 2.870~2.900(t, 2H, J=7.5Hz), 1.678~1.760(m, 2H), 1.342~ 1.410 (m, 4H), 0.896~0.925 (t, 3H, J=7Hz). MS (50eV) m / z (%): 360.9(43), 303.9(100), 271.9(7).
[0082] The above structural identification data show that the synthesized compound is indeed 2-(3-fluoro-4-isothiocyanatophenyl)-5-pentylthieno[3,2-b]thiophene.
[0083] The liquid crystal phase transition characteristic temperature was measured by DSC at a heating rate of 5°C / min, and the result was: C...
Embodiment 3
[0084] Example 3: Synthesis of 2-(3,5-difluoro-4-isothiocyanatophenyl)-5-butylthieno[3,2-b]thiophene:
[0085]
[0086] Butyryl chloride was used to replace valeryl chloride in step (1) of Example 1, and 2,6-difluoro-4-iodoaniline was used as a raw material to synthesize 2-(3,5-difluoro-4 -isothiocyanatophenyl)-5-butylthieno[3,2-b]thiophene.
[0087] Structure Identification: 1 H NMR (δ, CDCl 3 ): 7.411(s, 1H), 7.153~7.191(m, 2H), 6.947(s, 1H), 2.881~2.911(t, 2H, J=7.5Hz), 1.683~1.743(m, 2H), 1.389~ 1.464 (m, 2H), 0.940~0.969 (t, 3H, J=7.5Hz). MS (50 eV) m / z (%): 365.0 (49), 322.0 (100), 289.9 (8).
[0088] The above structural identification data show that the synthesized compound is indeed 2-(3,5-difluoro-4-isothiocyanatophenyl)-5-butylthieno[3,2-b]thiophene.
[0089] Using DSC to test the characteristic temperature of liquid crystal phase transition at a heating rate of 5°C / min, the result is: Cr73.53S A 124.68N129.02I. The monomer was dissolved in the basic formu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com