A kind of one-dimensional chiral cu(ii) chain complex and its preparation method and application
A complex and chiral technology, applied in the direction of 1/11 group organic compounds without C-metal bonds, organic chemistry methods, copper organic compounds, etc. Synthesis difficulties and other problems, to achieve the effect of convenient industrial scale production, mild reaction temperature and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of Chiral Organic Ligand LR or Enantiomer LS
[0051] Concrete preparation steps are as follows:
[0052] (1) Under nitrogen protection, with p-bromoacetophenone (1.99g, 10mmol) and p-pyridineboronic acid (1.23g, 10mmol) potassium carbonate (40.00mmol, 5.53g) and tetrakis-(triphenylphosphine) palladium (0.50 mmol, 0.57g), 80mL (EtOH:H2O:toluene=3:2:3) mixture was placed in a 250mL three-necked flask, and heated to reflux for 48h. Cooling, liquid separation, discarding the water phase, and evaporating the solvent under reduced pressure to obtain a crude product, which was separated by column chromatography (ethyl acetate, methanol) to obtain 2.58 g of a yellow solid, namely Intermediate A, yield: 80.1%.
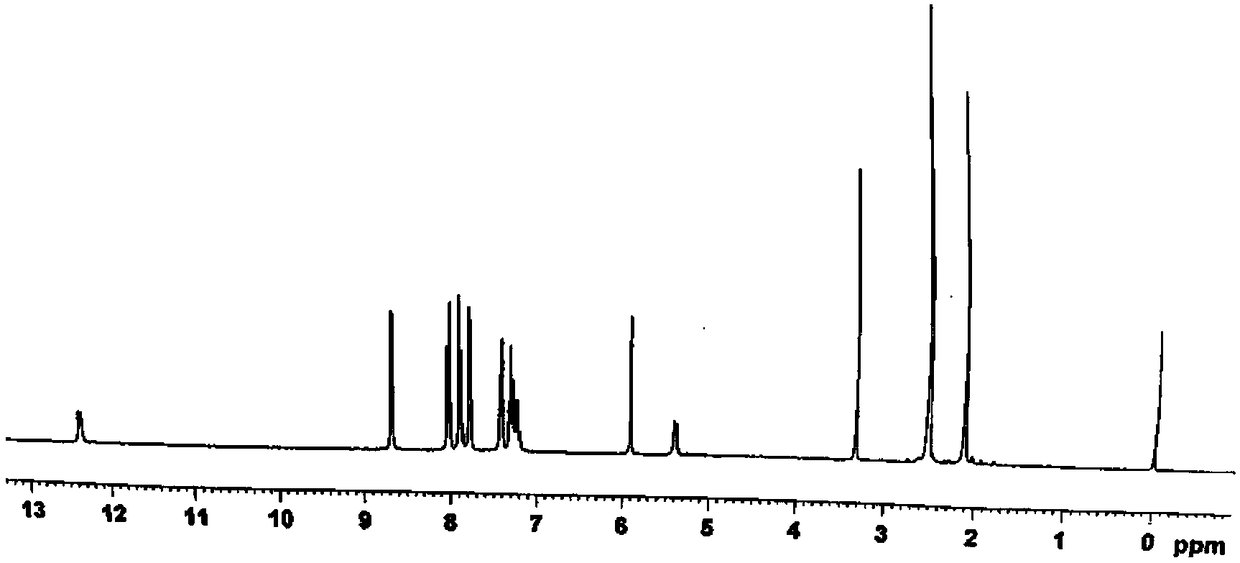
[0053] (2)N 2 Under protection, intermediate A (1.97g, 10mmol) and sodium hydride (0.28g, 11.64mmol) were placed in a 100ml three-necked flask, and 50ml of anhydrous THF was slowly added as a solvent, heated to 80°C, and stirred at a constant te...
Embodiment 2
[0056] Embodiment 2: the synthesis of chiral Cu (II) molecular building block
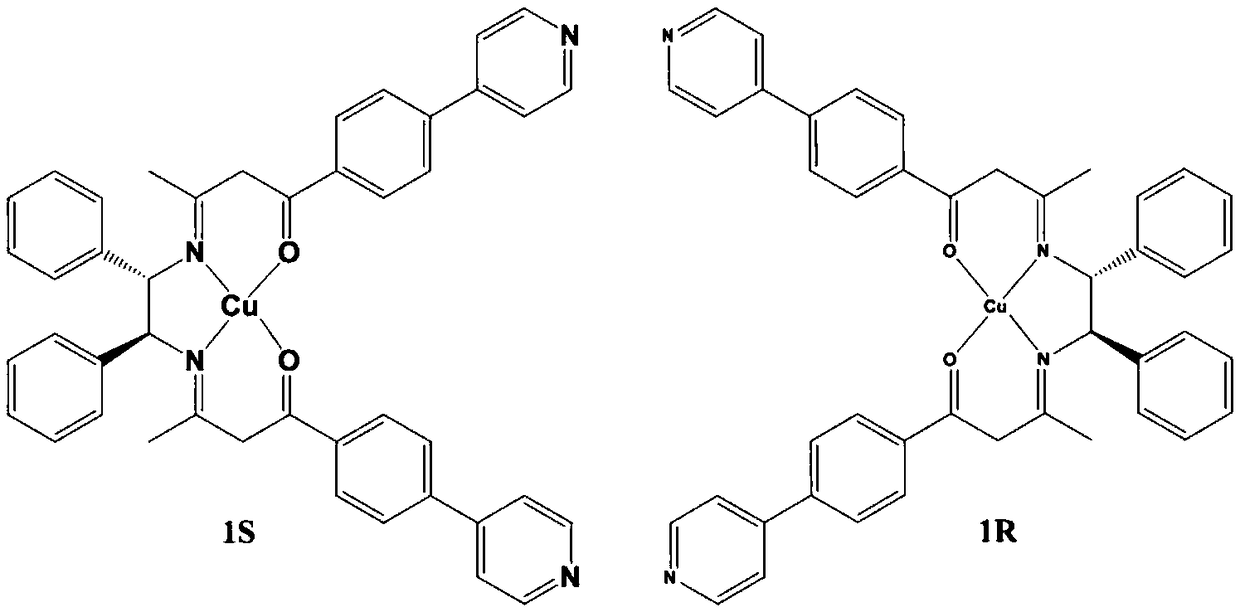
[0057] The organic ligand LR or LS (69.1mg, 0.10mmol) prepared in Example 1, and copper acetate (19.9mg, 0.10mmol) were dissolved in 5mL of methanol, and stirred at room temperature for 2 hours to obtain red blocky crystals [CuL]. Yield 75.7 mg, yield 81% (based on L).
[0058] We characterized the compound by the properties of IR and CD, the results are shown in Figure 6-9 .
Embodiment 3
[0059] Example 3: Synthesis of one-dimensional chiral Cu(II) chain complexes
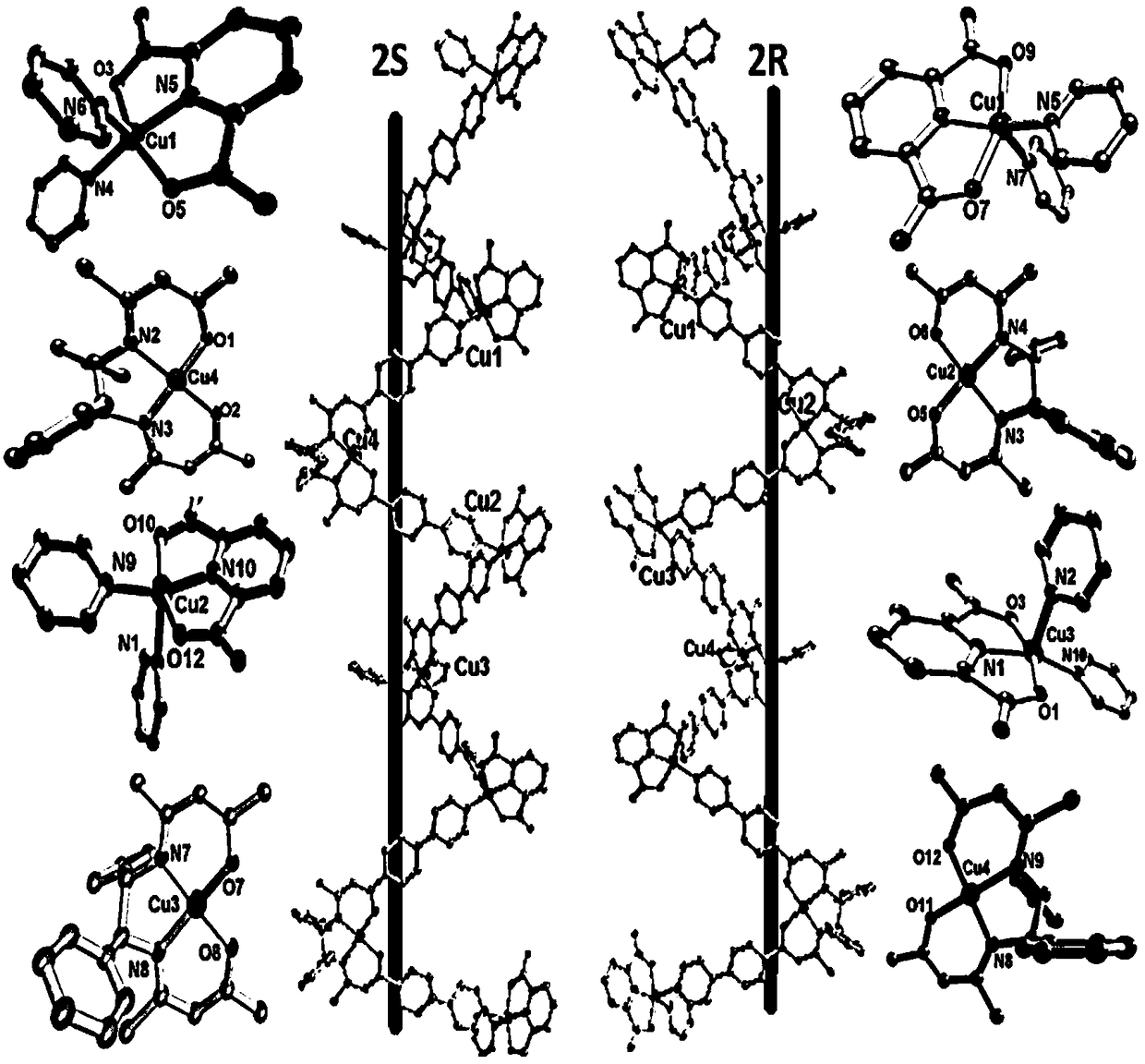
[0060] The chiral Cu molecular building block prepared in Example 2 (71.6mg, 0.1mmol), 1,5-dicarboxypyridine (16.7mg, 0.1mmol)) was dissolved in 2mL methanol, placed in a 5ml small test tube, 120 The temperature was kept at ℃ for 72 hours, and the temperature was lowered to room temperature after 50 hours of programming to obtain red blocky crystals, that is, one-dimensional chiral Cu(II) chain complexes, with a yield of 48.6 mg and a yield of 55% (based on L).
[0061] We characterized the compound by the properties of IR and CD, the results are shown in Figure 10-13 , it needs to be pointed out that the circular dichroism spectral properties of the one-dimensional chiral Cu(II) chain complexes 2R and 2S show negative Cotton effects at 300 and 410nm, and positive Cotton effects at 250nm and 330nm . The good mirror relationship in the CD spectrum indicates that the complexes 2R and 2S are enantio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com