Fibrous-protein-modified open-tubular column and application to monoclonal antibody isomer separation
A monoclonal antibody and fibrin technology, which is applied in the field of instrument analysis, can solve the problem of non-uniformity of monoclonal antibodies, and achieve the effects of improving separation effect, inhibiting adsorption, and mild preparation conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A fibrin-modified open-tubular column prepared by the following steps:
[0032] (1) Rinse the open column with sodium hydroxide solution (1M), deionized water, hydrochloric acid solution (1M) and deionized water in sequence, and dry it;
[0033] (2) Mix fibrinogen solution (2 mg / mL, 500 μL), thrombin solution (0.12 μM, 25 μL) and phosphate buffer (10 mM, pH 7.4, containing 475 μL of 0.15M sodium chloride), and quickly Inject it into a pretreated open-tubular column, store it in a thermostat at 37°C for 5 hours, then flush out excess reaction solution with nitrogen and dry it in an environment at 40°C under its protection. Finally, the column was washed with 10 mM phosphate (pH value 7.4) and deionized water, and dried under nitrogen protection at 40°C to obtain a fibrin-modified open-tube column.
Embodiment 2
[0035] The application of the fibrin modified open-tubular column prepared in Example 1 in the separation of 6 cetuximab isomers comprises the following steps:
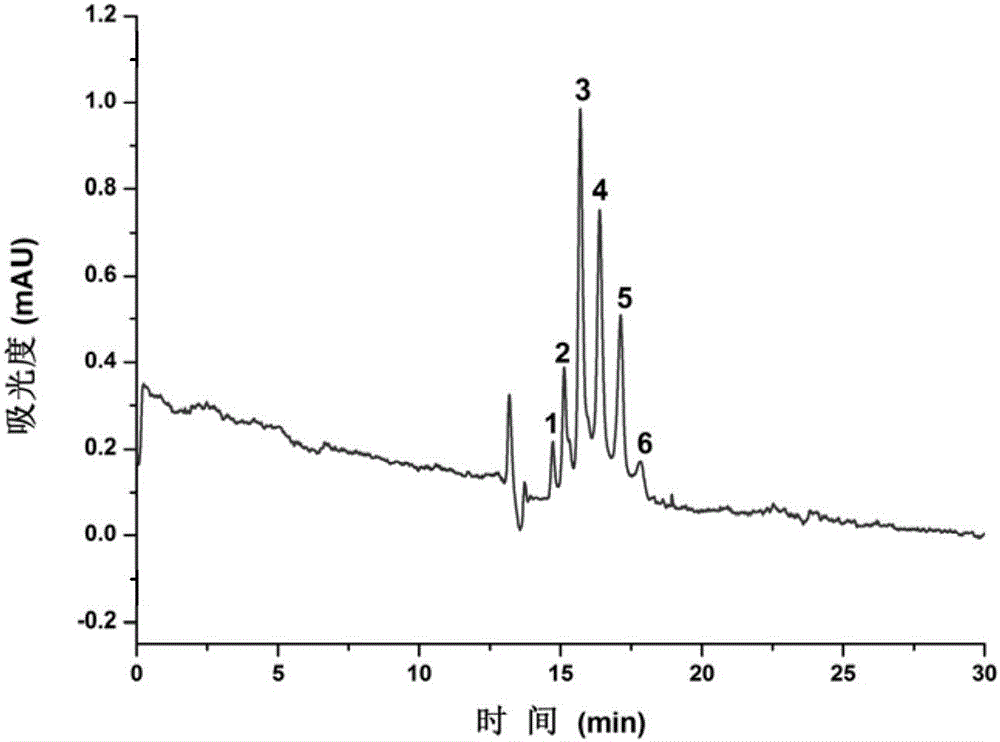
[0036] Dissolve cetuximab after centrifugation and dilution in water, and separate it by electrochromatography. The results are shown in figure 1 . The separation conditions are as follows: the inner diameter of the chromatographic column is 50 μm, the total length is 62.5 cm, the effective length is 37.0 cm, the mobile phase is 50 mM (pH 7.0) phosphate buffer, the separation voltage is 20 kV, the detection wavelength is 214 nm, and the sampling method is height Injection.
[0037] Depend on figure 1 It can be seen that the six different forms of isomers of cetuximab are well separated on the fibrin-modified open-tubular column, and the peak shape is sharp, indicating that the coated column can be effectively applied to cetuximab Identification and separation of mAb isoforms.
Embodiment 3
[0039] The application of the fibrin modified open-tubular column prepared in Example 1 in separating 5 kinds of rituximab isomers comprises the following steps:
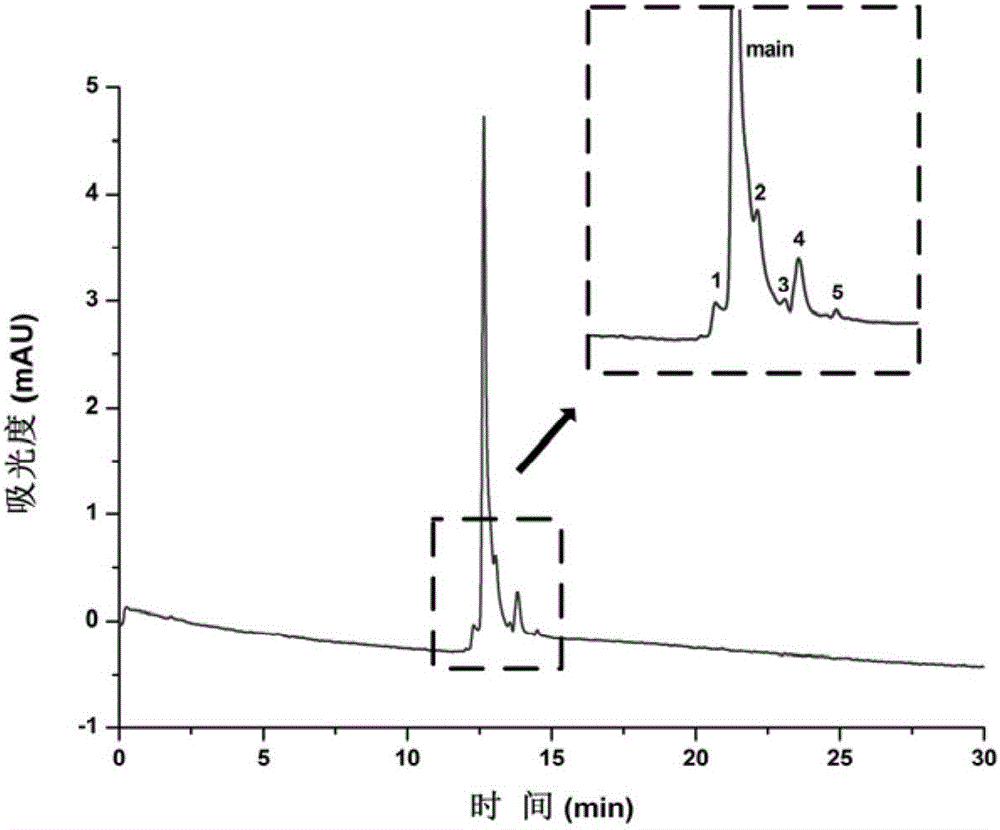
[0040] The centrifuged and diluted rituximab was dissolved in water and separated by electrochromatography. The results are shown in figure 2 . The separation conditions are as follows: the inner diameter of the chromatographic column is 50 μm, the total length is 62.5 cm, the effective length is 37.0 cm, the mobile phase is 50 mM (pH 7.0) phosphate buffer, the separation voltage is 20 kV, the detection wavelength is 214 nm, and the sampling method is height Injection.
[0041] Depend on figure 2 It can be seen that five different isomers of rituximab were successfully separated from the main peak, indicating that the coated column can be effectively applied to the identification and separation of rituximab isomers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com